Abstract
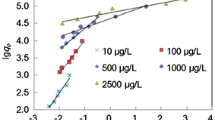
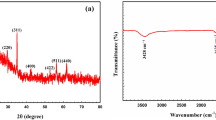
This study aims to investigate Cs removal by different external metals hexacyanoferrates MII–Fe PBA (MII: Co, Ni and Cu). Three PBAs were synthesized, characterized and followed by batch experiment testing and model fitting. Among the three PBAs, Cu–Fe PBA possessed the highest capacity 234.28 mg g−1, the highest Kd 2.8 × 106 mL g−1 and the fastest adsorption kinetics on Cs removal. Moreover, Cu–Fe PBA and Ni–Fe PBA were the more selective sorbents in coexisting ions study. Finally, the adsorption mechanism was proposed. This study provides new insights into the vacancies effect of the PBA structure and Cs adsorption application.







Similar content being viewed by others
References
Yoshida N, Kanda J (2012) Geochemistry. Tracking the Fukushima radionuclides. Science 336:1115–1116. https://doi.org/10.1126/science.1219493
Wang J, Zhuang S, Liu Y (2018) Metal hexacyanoferrates-based adsorbents for cesium removal. Coord Chem Rev 374:430–438. https://doi.org/10.1016/j.ccr.2018.07.014
Alby D, Charnay C, Heran M, Prelot B, Zajac J (2018) Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: synthesis and shaping, sorption capacity, mechanisms, and selectivity—a review. J Hazard Mater 344:511–530. https://doi.org/10.1016/j.jhazmat.2017.10.047
Ludi A., Güdel HU (1973) Structural chemistry of polynuclear transition metal cyanides. In: Inorganic chemistry. Structure and bonding, vol 14. Springer, Berlin. https://doi.org/10.1007/BFb0016869
Naidu G, Nur T, Loganathan P, Kandasamy J, Vigneswaran S (2016) Selective sorption of rubidium by potassium cobalt hexacyanoferrate. Sep Purif Technol 163:238–246. https://doi.org/10.1016/j.seppur.2016.03.001
Parajuli D, Takahashi A, Noguchi H, Kitajima A, Tanaka H, Takasaki M, Yoshino K, Kawamoto T (2016) Comparative study of the factors associated with the application of metal hexacyanoferrates for environmental Cs decontamination. Chem Eng J 283:1322–1328. https://doi.org/10.1016/j.cej.2015.08.076
Mimura H, Lehto J, Harjula R (1997) Ion exchange of cesium on potassium nickel hexacyanoferrate (II)s. J Nucl Sci Technol 34:484–489. https://doi.org/10.1080/18811248.1997.9733695
Takahashi A, Minami N, Tanaka H, Sue K, Minami K, Parajuli D, Lee K-M, Ohkoshi S-i, Kurihara M, Kawamoto T (2015) Efficient synthesis of size-controlled open-framework nanoparticles fabricated with a micro-mixer: route to the improvement of Cs adsorption performance. Green Chem 17:4228–4233. https://doi.org/10.1039/c5gc00757g
Yang H-M, Park CW, Kim I, Yoon I-H (2020) Hollow flower-like titanium ferrocyanide structure for the highly efficient removal of radioactive cesium from water. Chem Eng J 392. https://doi.org/10.1016/j.cej.2019.123713
Lee EFT, Streat M (1983) Sorption of caesium by complex hexacyanoferrates. v. A comparison of some cyanoferrates. J Chem Tech Biotechnol 33:333–338. https://doi.org/10.1002/jctb.504330204
Ishizaki M, Akiba S, Ohtani A, Hoshi Y, Ono K, Matsuba M, Togashi T, Kananizuka K, Sakamoto M, Takahashi A, Kawamoto T, Tanaka H, Watanabe M, Arisaka M, Nankawa T, Kurihara M (2013) Proton-exchange mechanism of specific Cs+ adsorption via lattice defect sites of Prussian blue filled with coordination and crystallization water molecules. Dalton Trans 42:16049–16055. https://doi.org/10.1039/c3dt51637g
Yu Z-E, Cheng H, Lyu Y, Liu Y, Zhou J, Chen R, Guo B (2021) A vacancy-free sodium manganese hexacyanoferrate as cathode for sodium-ion battery by high-salt-concentration preparation. J Alloys Compd 887. https://doi.org/10.1016/j.jallcom.2021.161388
Takahashi A, Tanaka H, Minami K, Noda K, Ishizaki M, Kurihara M, Ogawa H, Kawamoto T (2018) Unveiling Cs-adsorption mechanism of Prussian blue analogs: Cs(+)-percolation via vacancies to complete dehydrated state. RSC Adv 8:34808–34816. https://doi.org/10.1039/c8ra06377j
Vincent T, Vincent C, Guibal E (2015) Immobilization of metal hexacyanoferrate ion-exchangers for the synthesis of metal ion sorbents—a mini-review. Molecules 20:20582–20613. https://doi.org/10.3390/molecules201119718
Aguila D, Prado Y, Koumousi ES, Mathoniere C, Clerac R (2016) Switchable Fe/Co Prussian blue networks and molecular analogues. Chem Soc Rev 45:203–224. https://doi.org/10.1039/c5cs00321k
Kim M, Park J-H, Lim J-M, Kim H, Kim S (2021) Conventional and photoinduced radioactive 137Cs removal by adsorption on FeFe, CoFe, and NiFe Prussian blue analogues. Chem Eng J 405. https://doi.org/10.1016/j.cej.2020.126568
Cano A, Rodríguez-Hernández J, Reguera L, Rodríguez-Castellón E, Reguera E (2019) On the scope of XPS as sensor in coordination chemistry of transition metal hexacyanometallates. Eur J Inorg Chem 2019:1724–1732. https://doi.org/10.1002/ejic.201801556
Trung ND, Ping N, Dan HK (2022) Synthesis, characterization, and the effectiveness of cobalt hexacyanoferrate nanoparticles in Cs+ adsorbent application. Nanotechnol Environ Eng. https://doi.org/10.1007/s41204-022-00265-x
Ke Y, Li Y, Zhu L, Zhou Y, Liu D (2020) Rapid enrichment of cesium ions in aqueous solution by copper ferrocyanide powder. SN Appl Sci 2. https://doi.org/10.1007/s42452-020-2337-8
Bhatt P, Banerjee S, Mukadam MD, Jha P, Navaneethan M, Yusuf SM (2022) Enhanced hydrogen adsorption in alkali metal based copper hexacyanoferrate Prussian blue analogue nanocubes. J Power Sources 542. https://doi.org/10.1016/j.jpowsour.2022.231816
Liu J, Li X, Rykov AI, Fan Q, Xu W, Cong W, Jin C, Tang H, Zhu K, Ganeshraja AS, Ge R, Wang X, Wang J (2017) Zinc-modulated Fe–Co Prussian blue analogues with well-controlled morphologies for the efficient sorption of cesium. J Mater Chem A 5:3284–3292. https://doi.org/10.1039/c6ta10016c
Vincent T, Vincent C, Barré Y, Guari Y, Le Saout G, Guibal E (2014) Immobilization of metal hexacyanoferrates in chitin beads for cesium sorption: Synthesis and characterization. J Mater Chem A 2. https://doi.org/10.1039/C4TA01128G
Yoon CM, Ryu J, Yun J, Kim YK, Jang J (2018) Synthesis of hierarchical Silica/Titania hollow nanoparticles and their enhanced electroresponsive activity. ACS Appl Mater Inter 10:6570–6579. https://doi.org/10.1021/acsami.7b18895
Bu F-X, Hu M, Zhang W, Meng Q, Xu L, Jiang D-M, Jiang J-S (2015) Three-dimensional hierarchical Prussian blue composed of ultrathin nanosheets: enhanced hetero-catalytic and adsorption properties. Chem Commun 51:17568–17571. https://doi.org/10.1039/C5CC06281K
Zhang N, Kawamoto T, Jiang Y, Takahashi A, Ishizaki M, Asai M, Kurihara M, Zhang Z, Lei Z, Parajuli D (2019) Interpretation of the role of composition on the inclusion efficiency of the monovalent cations onto cobalt hexacyanoferrate. Chem Eur J 25. https://doi.org/10.1002/chem.201900097
Nordstrand J, Toledo-Carrillo E, Kloo L, Dutta J (2022) Sodium to cesium ions: a general ladder mechanism of ion diffusion in prussian blue analogs. Phys Chem Chem Phys 24:12374–12382. https://doi.org/10.1039/d2cp01156e
Zhang H, Zhao X, Wei J, Li F (2015) Removal of cesium from low-level radioactive wastewaters using magnetic potassium titanium hexacyanoferrate. Chem Eng J 275:262–270. https://doi.org/10.1016/j.cej.2015.04.052
Zhang H, Qi J, Liu F, Wang Z, Ma X, He D (2021) One-pot synthesis of magnetic Prussian blue for the highly selective removal of thallium(I) from wastewater: Mechanism and implications. J Hazard Mater 423:126972. https://doi.org/10.1016/j.jhazmat.2021.126972
Lee IH, Kuan Y-C, Chern J-M (2007) Equilibrium and kinetics of heavy metal ion exchange. J Chin Inst Chem Eng 38:71–84. https://doi.org/10.1016/j.jcice.2006.11.001
Singh S, Townsend T, Mazyck D, Boyer T (2011) Equilibrium and intra-particle diffusion of stabilized landfill leachate onto micro- and meso-porous activated carbon. Water Res 46:491–499. https://doi.org/10.1016/j.watres.2011.11.007
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interface Sci 286:90–100. https://doi.org/10.1016/j.jcis.2005.01.007
Alamudy HA, Cho K (2018) Selective adsorption of cesium from an aqueous solution by a montmorillonite-prussian blue hybrid. Chem Eng J 349:595–602. https://doi.org/10.1016/j.cej.2018.05.137
Chang L, Chang S, Chen W, Han W, Li Z, Zhang Z, Dai Y, Chen D (2016) Facile one-pot synthesis of magnetic Prussian blue core/shell nanoparticles for radioactive cesium removal. RSC adv 6:96223–96228. https://doi.org/10.1039/C6RA17525B
Vincent C, Hertz A, Vincent T, Barré Y, Guibal E (2014) Immobilization of inorganic ion-exchanger into biopolymer foams—application to cesium sorption. Chem Eng J 236:202–211. https://doi.org/10.1016/j.cej.2013.09.087
Chen X, Li Y, Zhu L, Ke Y, Wang X, Yang Y (2020) High-efficiency continuous enrichment of cesium ions using CuFC composite microspheres: dynamic adsorption and mechanism analysis. J Radioanal Nucl Chem 326:959–973. https://doi.org/10.1007/s10967-020-07378-0
Naeimi S, Faghihian H (2017) Performance of novel adsorbent prepared by magnetic metal-organic framework (MOF) modified by potassium nickel hexacyanoferrate for removal of Cs+ from aqueous solution. Sep Purif Technol 175:255–265. https://doi.org/10.1016/j.seppur.2016.11.028
Lehto J, Harjula R, Wallace J (1987) Absorption of cesium on potassium cobalt hexacyanoferrate(II). J Radioanal Nucl Chem 111:297–304. https://doi.org/10.1007/BF02072863
Michel C, Barré Y, de Dieuleveult C, Grandjean A, De Windt L (2015) Cs ion exchange by a potassium nickel hexacyanoferrate loaded on a granular support. Chem Eng Sci 137:904–913. https://doi.org/10.1016/j.ces.2015.07.043
Lee EFT, Streat M (1983) Sorption of caesium by complex hexacyanoferrates iii. a study of the sorption properties of potassium copper ferrocyanide. J Chem Tech Biotechnol 33:80–86. https://doi.org/10.1002/jctb.504330203
Cattermull J, Pasta M, Goodwin AL (2021) Structural complexity in Prussian blue analogues. Mater Horizons 8:3178–3186. https://doi.org/10.1039/d1mh01124c
Acknowledgements
The research leading to these results received funding from the Chinese Academy of Sciences Pioneer “Hundred Talents Program” Young Talents (Class C). This publication reflects only the authors’ views, exempting the community from any liability.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nong, C., Li, X. & Xu, J. Systematic effect of different external metals of hexacyanoferrates on cesium adsorption behavior and mechanism. J Radioanal Nucl Chem 332, 1263–1275 (2023). https://doi.org/10.1007/s10967-022-08721-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08721-3