Abstract
This study was the first to determine the effectiveness of radon concentration measurement in water using the degassing method as a screening method based on the liquid scintillation counter method. The degassing method used two radon monitors (AlphaGUARD and RAD7) and two monitor-attached degassing devices (AquaKIT and RAD H2O). These were effective screening methods based on the WHO guideline value of 100 Bq L−1. The screening values confirmed that a combination of the AlphaGUARD and AquaKIT was preferred. The results are useful for radon measurement for exposure control in drinking water and environmental in-situ field measurement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radon (222Rn) is a naturally occurring radioactive gas that is inert, colorless, and odorless. Radon and its parent nuclide, 226Ra, belong to the uranium decay series and have a half-life of 3.82 days [1]. The dissolution water/gas partition coefficient, K, of radon is 0.260 at 20 °C [2]. Environmental measurement of groundwater radon concentration has been used as a tracer and precursor in several hydrogeological and geological applications, such as earthquake predictions and geochemical explorations. [3].
According to the United Nations Scientific Committee on the Effects of Radon on the Environment (UNSCEAR), estimates of the radon transfer coefficient from water to indoor air-derived from existing published data elucidated a mean value of 10−4 [4]. Currently, the World Health Organization (WHO) Guidelines for Drinking Water Quality do not provide recommended radon levels because it is considered more appropriate to measure radon concentrations in indoor air than in drinking water [5]. However, the WHO's 2004 guidelines on radon in drinking water state that radon levels in drinking water should be controlled if it exceeds 100 Bq L−1 (hereinafter referred to as the WHO guideline value) in public water supplies [6]. The implementation of the EC Directive 2013/51/EURATOM on October 22, 2013 has also led to limit values being set for the concentration of radon in water for human consumption [7].
Theory
The liquid scintillation counting (LSC) method is the most common method for radon detection, which extracts radon in water into an organic scintillator and measures the radon concentration [8]. However, the use of organic scintillators (harmful organic solvents) should be avoided because they are difficult to transport by airplane to different parts of the world for measurements. In addition, the LSC method is not suitable for in-situ measurements, which are often conducted for environmental measurements. Therefore, degassing devices that can transfer radon from the aqueous phase to the air phase through aeration have attracted attention for use with conventional radon-in-air monitors which measure radon concentrations in the air [9]. Using a radon-in-air monitoring device equipped with this degassing system can measure the radon concentration in water without using an organic scintillator.
On the other hand, Kozak et al. [10] reported that the AlphaGUARD and AquaKIT (AlphaGUARD (ionization chamber) equipped with an AquaKIT system (Bertin Technologies SAS, France)) [11] provided good results compared to the LSC method. However, the results of the RAD7 and RAD H2O (RAD7 (semiconductor detector) equipped with a RAD H2O system (Durridge Company, USA)) [12] were lower than those of the LSC method [13,14,15]. Higuchi et al. [14] found that RAD7 and RAD H2O with leak prevention detected radon concentrations of 9.0–89 Bq L−1 under a relative humidity of ≤ 6% in the inner chamber of the RAD7 device. Furthermore, Takakaze et al. [16] found that RAD7 and AquaKIT (RAD H2O in the RAD7 and RAD H2O was replaced with the AquaKIT) detected radon concentrations of 41–129 Bq L−1 under a relative humidity of ≤ 6% in the inner chamber of the RAD7 device.
The degassing method is recommended by the International Organization for Standardization (ISO) [9] to measure radon concentration in water. However, the United States Environmental Protection Agency (US EPA) do not adopt the degassing method to measure radon in water because it requires advanced techniques [17, 18]. Only the LSC method is recognized by both the ISO [9] and US EPA [18].
After an accident at Tokyo Electric Power Company’s Fukushima Daiichi Nuclear Power Station which contaminated a wide range of soil and food products with radioactive materials, testing procedures were initiated using the index values of food etc. published by the Nuclear Safety Commission as provisional regulation values. For this purpose, the Japanese Ministry of Health, Labor, and Welfare (MHLW) established a screening method that reliably identifies samples with radioactive concentrations lower than the provisional regulation value [19]. As a result, it has become possible to perform the following assessments: (1) confirming the effectiveness of a given screening device; and (2) confirming screening values to identify specimens that are reliably lower than the provisional regulation values. In addition, when the result of the screening device measurement is a value higher than the screening value, further precise measurements are performed to determine the test result.
Therefore, considering these imminent problems regarding the quality of drinking water, in this study, we measured the radon concentration using the AlphaGUARD and AquaKIT and compared the results with those of the LSC method. In this study, a desiccant was also attached to reduce the damage caused by exposing the inside of the AlphaGUARD to high humidity. This study aimed to compare the screening values of the degassing system with previous studies [14, 16] and provide recommendations regarding the effectiveness of each method.
Experimental
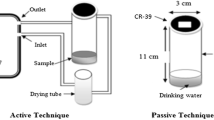
A degassing method using the AlphaGUARD and AquaKIT (BertinInstruments, France) was used to measure radon concentrations in water (Fig. 1) and was compared to an LSC method as the reference. Groundwater previously sampled at Kobe Pharmaceutical University in Kobe Japan had a radon concentration of ~ 200 Bq L−1, our research team reported [20]. The groundwater was diluted with tap water to various concentrations to make experimental samples. One test sample was measured using the degassing method, and three reference samples were measured using the LSC method.
Figure 1 shows the AlphaGUARD and AquaKIT in which the AquaKIT, a filter, and the AlphaGUARD were placed in a closed loop. The AquaKIT consisted of degassing and security vessels. A tube containing 5 g of desiccant (Drierite, W. A. Hammond Drierite Co. Ltd., 10–20 mesh) was connected to the glass tube of the security vessel to reduce humidity inside of the AlphaGUARD.
The experimental sample (0.1 L) was poured into the degassing vessel as a test sample. The pump was used to aerate the sample and transfer the radon to the entire AlphaGUARD at an air flow rate of 0.3 L min−1 for 10 min. After stopping the pump, the radon concentration was measured four times for 5 min each (20 min total). The radon concentration, CR1 (Bq L−1), was the average of the four original display values measured with the AlphaGUARD, which was calibrated using the standard radon gas in a radon exposure chamber located at Hirosaki University in Aomori, Japan [21]. The calibration constant of the AlphaGUARD was determined to be f1 = 0.932. The background value, CB1 (Bq L−1), of the device was measured using nitrogen gas without radon. From this CR1, we calculated the radon concentration in the water, CR2 (Bq L−1), using Eq. (1) [11].
where K is the water/gas partition coefficient of radon, VW (L) is the volume of the water sample (0.1 L), and VA (L) is the air volume of the system. The VA was obtained by subtracting the volume of the desiccant (0.014 L) and VW from the volume of the system (1.122 L) [11]. The K was calculated using the mole fraction solubility at the sample water temperature using the formula given by Gevantman [2].
The values of CR2 were indicated by high values for the reduced humidity inside of the AlphaGUARD using the desiccant. Radon and radon progeny nuclides (main nuclide: 218Po) were present in the detector. Radon progeny nuclides became positively charged ions due to their metallic nature. Decreased humidity reduced the number of water particles attached to the ions, and the counting efficiency of alpha rays emitted from the ions was thought to increase due to reduced shielding by the water particles. Therefore, the correction coefficient was determined to be f2 = 1.30 using water samples with a radon concentration of ~ 150 Bq L−1. The radon concentration, CR (Bq L−1), of the test sample was converted from CR2 by f2 using Eq. (2).
In the LSC method, three reference samples were prepared using the experimental sample. The reference sample (10 mL) was added to a highly efficient mineral oil scintillation cocktail (10 mL; PerkinElmer Inc., USA) in a 20 mL glass LSC vial (PerkinElmer Inc., USA). The vials were shaken for 30 s to extract radon from the water into the scintillator and left for ~ 4 h until radon and its descendants achieved radiation equilibrium. The average counting ratio of the three reference samples was measured using the integral counting method of the LSC method (2300TR, PerkinElmer Inc., USA) for 60 min. The radon concentration, CS (Bq L−1), was converted from the average counting rate of the three reference samples [8].
Screening method
To determine the screening criteria of the WHO guideline value (100 Bq L−1), numeric values for the following two criteria were established based on the MHLW screening method [19]. In the first criterion, the limit of detection (LOD) must be < 25 Bq L−1 (i.e., one-quarter of the WHO guideline value, 100 Bq L−1). In the second criterion, an effective screening value was established. Using experimental data, a linear regression (black line in Fig. 2) and a 99% prediction interval (99% PI; grey zone in Fig. 2) were determined. In this study, the 99% prediction interval was calculated in IBM SPSS Statistics V.22.0.0 (SPSS Inc., Chicago, IL, USA). The y value in Fig. 2a is a candidate value of the screening value used for acceptance or rejection when the degassing method was used for screening. When Cs = x1 in Fig. 2a (the WHO guideline value of 100 Bq L−1), CR is mostly (99.5%) larger than the y value, and the sample is rejected. When Cs = x2 in Fig. 2a, CR is mostly (99.5%) smaller than the y value, and the sample is accepted. If the y and x2 values are > 50 Bq L−1 (i.e., half of the WHO guideline value), the screening method is established. If the degassing method met the above two criteria, the y value can be used for acceptance or rejection as the screening value.
Assessment of the screening value of the three degassing methods. The black line is regression line, and the grey zone is the 99% prediction interval. a the AlphaGUARD and AquaKIT, x1: the WHO guideline value, y: the test screening value, x2: the reference screening value, b the RAD7 and RAD H2O, c the RAD7 and AquaKIT
Results and discussion
A total of 59 experiments were conducted in the radon concentration range of 7–117 Bq L−1.
Lower limit of detection (LOD) and quantification (LOQ)
The LOQ was determined using the difference between the values obtained by the degassing and reference methods. The criteria for quantitative measurement were met at the 95% confidence interval (95% CI) of Individual Percent Error (IPE%), which existed between − 25 and 25% [22, 23]. Figure 3 shows the relationship between the CS and IPE% values of the AlphaGUARD and AquaKIT used to determine the accuracy. When the 95% CI of IPE% (the error bars in Fig. 3) is within the criteria (the grey zone in Fig. 3) [22, 23], the verification measurement is considered accurate. The verification measurement of the AlphaGUARD and AquaKIT showed a minimum quantifiable value of 28 Bq L−1. The LOD, considered to be 1/3 of the LOQ [24], was 9.3 Bq L−1. Similarly, Table 1 shows the LOQs and the LODs of other degassing methods obtained in a previous study [16]. All degassing methods in Table 1 met the first criterion of the screening method because the LOD was < 25 Bq L−1.
Effectiveness of the degassing method as a screening method
The data exceeding the above-estimated LOQ was used as the analysis data. Using these data, a linear regression (black line in Fig. 2) and a 99% prediction interval (99% PI; grey zone in Fig. 2) were determined. Figure 2a shows the results of the AlphaGUARD and AquaKIT. Figure 2b shows the results from the RAD7 and RAD H2O method, which were taken from Takakaze et al. [16] (see Fig. 4 in [16]). The results were below 100 Bq L−1. In order to determine the screening value of the reference value (100 Bq L−1), results exceeding 100 Bq L−1 were necessary. Therefore, three results above 100 Bq L−1 were added to the experimental results from Takakaze et al. [16]. Figure 2c shows the results of the RAD7 and AquaKIT method which were also taken from Takakaze et al. [16] (see Fig. 2 in [16]). The value of x2 in Fig. 2c was changed to 83 Bq L−1 based on our recalculation, although it was initially noted as 84 Bq L−1 by Takakaze et al. [16].
Regarding the second criterion of the AlphaGUARD and AquaKIT, when the value of x1 in Fig. 2a met the WHO guideline value (100 Bq L−1), y in Fig. 2a was 92 Bq L−1. The value of x2 in Fig. 2a was 87 Bq L−1. All degassing methods in Table 1 met the second criterion of the screening method because their the y and x2 values were > 50 Bq L−1.
The effectiveness of all degassing systems as a screening method was confirmed by comparing them with the LSC method as a reference, and the results are shown in Table 1. The screening values of the AlphaGUARD and AquaKIT and the RAD7 and AquaKIT were closer to the WHO guideline value (100 Bq L−1) than that of the RAD7 and RAD H2O. Furthermore, for the RAD7 and RAD H2O, the requirement to leak prevention was a disadvantage. The LOD of the AlphaGUARD and AquaKIT were smaller than that of the RAD7 and AquaKIT. Therefore, when screening for radon concentration in water using the degassing method, it is recommended to use the AlphaGUARD and AquaKIT.
Conclusions
Radon concentration in water is typically measured using the LSC method; however, this method uses a harmful organic solvent as a liquid scintillator and is difficult to use in situ. Therefore, the degassing method is a viable alternative because it allows the measurable range to be expanded. Water samples with a radon concentration of > 28 Bq L−1 could be quantitatively measured using the AlphaGUARD and AquaKIT. By attaching a desiccant to the AlphaGUARD and AquaKIT, the damage to the AlphaGUARD caused by high humidity was reduced in this study. Further, the other two studied degassing systems were effective. Based on the above, all degassing systems in Table 1 were effective as screening devices because they satisfied two criteria.: first, the LOD were < 25 Bq L−1, and second, the the y and x2 values in Fig. 2a were > 50 Bq L−1 (50% of the WHO’s guideline value). In this study, the LOQs were obtained from actual values, and the LOD was determined as 1/3 of the LOQ. The screening values of the AlphaGUARD and AquaKIT and the RAD7 and AquaKIT were closer to the WHO guideline value (100 Bq L−1) than that of the RAD7 and RAD H2O. Furthermore, the requirement for the RAD7 and RAD H2O to leak prevention was a disadvantage. The detection limits of the AlphaGUARD and AquaKIT were smaller than the RAD7 and AquaKIT. Therefore, when screening for radon concentration in water using the degassing method, it is recommended to use AlphaGUARD and AquaKIT with a desiccant.
References
Baskaran M (2016) Radon: a tracer for geological, geophysical and geochemical studies. Springer, Cham
Gevantman LH (2013) Chapter 5 Solubility of selected gases in water. In: Haynes WM, Lide DR, Bruno TJ (eds) CRC handbook of chemistry and physics, 94th edn. CRC Press, Boca Raton, pp 5-149–150
Sukanya S, Noble J, Joseph S (2022) Application of radon (222Rn) as an environmental tracer in hydrogeological and geological investigations: an overview. Chemosphere 303:135141. https://doi.org/10.1016/j.chemosphere.2022.135141
United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) (2000) 2000 Report to the General Assembly, with scientific Annexes, Volume I Sources and effects of ionizing radiation. United Nations Scientific Committee on the Effects of Atomic Radiation. United Nations Publication, New York. https://www.unscear.org/unscear/uploads/documents/publications/UNSCEAR_2000_Annex-B.pdf. Accessed 1 Aug 2022
World Health Organization (WHO) (2021) Radon and health. https://www.who.int/news-room/fact-sheets/detail/radon-and-health. Accessed 25 July 2021
WHO (2004) Guidelines for drinking-water quality, 3rd (ed), volume 1 Recommendations; Microbial Fact Sheets. WHO, Geneva, p 207
Euratom (2013) Council Directive 2013/51/Euratom of 22 October 2013 laying down requirements for the protection of the health of the general public with regard to radioactive substances in water intended for human consumption. OJ L 296, 7.11.2013: pp 12–21. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013L0051. Accessed 25 July 2021
Tanaka R, Araki S, Yasuoka Y, Mukai T, Ohnuma S, Ishikawa T, Fukuhori N, Sanada T (2013) A simplified method for improved determination of radon concentration in environmental water samples. Radioisotopes 62:423–438
International Organization for Standardization (ISO) (2020) Water quality—radon-222—part 3: test method using emanometry (SIST EN ISO 13164-3:2020)
Kozak K, Kozłowska B, Przylibski TA, Mazur J, Adamczyk-Lorenc A, Mamont-Cieśla K, Stawarz O, Dorda J, Kłos B, Janik M, Kochowska E (2012) Intercomparison measurements of 222Rn concentration in water samples in Poland. Radiat Meas 47:89–95
Bertin Technologies SAS (Saphymo GmbH) (2009) AlphaGUARD: the reference in professional radon measurement. Saphymo GmbH, Frankfurt
Durridge Company Inc (2020) RAD H2O, Radon in water accessory for the RAD7, user manual. Durridge Company Inc, MA USA
Stojković I, Tenjović B, Nikolov J, Vesković M, Mrđa D, Todorović N (2015) Improvement of measuring methods and instrumentation concerning 222Rn determination in drinking waters—RAD7 and LSC technique comparison. Appl Radiat Isot 98:117–124
Higuchi S, Kamishiro Y, Ishihara M, Yasuoka Y, Mori Y, Hosoda M, Iwaoka K, Tokonami S, Takahashi R, Janik M, Muto J, Nagahama H, Mukai T (2019) Evaluation of a radon air monitor in the measurement of radon concentration in water in comparison with a liquid scintillation counter. Radiat Prot Dosim 184:426–429
Ishikawa T, Yasuoka Y, Narazaki Y, Tokonami S, Ishii T, Suda H, Yamada Y (2004) Comparison of instruments for measuring radon in groundwater. Radioisotopes 53:133–140
Takakaze Y, Yasuoka Y, Higuchi S, Matsumoto M, Hosoda M, Tokonami S, Iwaoka K, Mukai T (2021) Measurement of radon concentration in water using a radon-in-air monitor. In: Bessho K, Matsumura H, Yoshida G (eds) Proceeding of the 22nd workshop on environmental radioactivity (KEK proceedings 2021–2), Tsukuba, Ibaraki (Japan), 10–12 Mar 2021, High Energy Accelerator Research Organization, Tsukuba, Japan, pp 118–124
United States Environmental Protection Agency (US EPA), (1999). Federal Register. Radon-222: Propose rules vol. 64, no. 211/Tuesday, November 2. https://archive.epa.gov/water/archive/web/html/regulations.html. Accessed 15 Aug 2022
US EPA, Proposed radon in drinking water regulation. https://www.epa.gov/dwreginfo/radionuclides-rule. Accessed 15 Aug 2022
The Japanese Ministry of Health, Labour and Welfare (MHLW) (2012) Concerning the partial revision of the screening method for radioactive cesium in food products. MHLW, Tokyo, Japan. https://www.mhlw.go.jp/english/topics/2011eq/dl/food-120821_3%20(002).pdf. Accessed 16 Dec 2022
Yasuoka Y, Shinogi M (2000) Determination of radon concentrations in natural water in the Rokko area (Japan) and evaluation of its effective dose. Radioisotopes 49:551–557
Pornnumpa C, Oyama Y, Iwaoka K, Hosoda M, Tokonami S (2018) Development of radon and thoron exposure systems at Hirosaki University. Radiat Environ Med 7:13–20
The American Association of Radon Scientists and Technologists (AARST) and the American National Standards Institute (ANSI) (2014) Protocol for conducting measurements of radon and radon decay product in homes (MAH-2014). AARST, North Carolina
AARST and ANSI (2015) Performance specifications for instrumentation systems designed to measure radon gas in air (MS-PC 2015). AARST, North Carolina
Koike R, Mizuno Y, Koike K (2012) Methods for determining the limit of detection (LOD) and the limit of quantitation (LOQ) using the guideline titled “validation of analytical methods used in residue depletion studies.” Ann Rep Natl Vet Assay Lab 50:131–139 (in Japanese with English abstract)
Acknowledgements
This work was supported by Environmental Radioactivity Research Network Center (ERAN: F-22-45).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsumoto, M., Yasuoka, Y., Takakaze, Y. et al. Evaluation of radon concentration measurements in water using the radon degassing method. J Radioanal Nucl Chem 332, 167–172 (2023). https://doi.org/10.1007/s10967-022-08698-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08698-z