Abstract
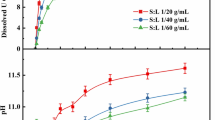
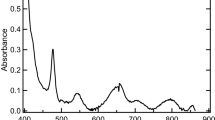
The dissolution behaviors of uranium oxides (UO2, U3O8, and UO2(O2)·4H2O) and the dissolved species of uranium in the carbonate-peroxide solution were investigated by UV–vis absorption spectrometry and interpreted by a theoretically calculated spectrum. The results indicate that the same dominant species (UO2(O2)(CO3)24−) in the solution was observed in spite of the differences among the three uranium oxides. Additionally, the dissolution of UO2(O2)·4H2O was found to be occurring much more rapidly in carbonate solution than those of UO2 and U3O8. The concentration of dissolved uranium for UO2(O2)·4H2O in the carbonate-peroxide solution could reach about 300 g/L.





Similar content being viewed by others
References
Nash KL, Lumetta GJ (2011) Advanced separation techniques for nuclear fuel reprocessing and radioactive waste treatment. Woodhead Publishing Limited, Sawston
Taylor R, Nash K, Nilsson M, Poinssot C, Koppelman B (2015) Reprocessing and recycling of spent nuclear fuel. nuclear energy encyclopedia: science, technology, and applications
Stepanov SI, Boyarintsev AV (2022) Reprocessing of spent nuclear fuel in carbonate media: Problems, achievements, and prospects. Nucl Eng Technol. https://doi.org/10.1016/j.net.2022.01.009
Stepanov SI, Boyarintsev AV, Vazhenkov MV, Myasoedov BF, Nazarov EO, Safiulina AM, Tananaev IG, So HV, Chekmarev AM, Civadze AY (2011) CARBEX process, a new technology of reprocessing of spent nuclear fuel. Russ J Gen Chem. 81(9):1949–1959. https://doi.org/10.1134/S1070363211090404
Boyarintsev AV, Stepanov SI, Kostikova GV, Zhilov VI, Chekmarev AM, Tsivadze AY (2019) Reprocessing of simulated voloxidized uranium-oxide SNF in the CARBEX process. Nucl Eng Technol 51(7):1799–1804. https://doi.org/10.1016/j.net.2019.05.020
Soderquist C, Hanson B (2010) Dissolution of spent nuclear fuel in carbonate-peroxide solution. J Nucl Mater 396(2–3):159–162. https://doi.org/10.1016/j.jnucmat.2009.11.001
Kim KW, Chung DY, Yang HB, Lim JK, Lee EH, Song KC, Song K (2009) A conceptual process study for recovery of uranium alone from spent nuclear fuel by using high-alkaline carbonate media. Nucl Technol 166(2):170–179. https://doi.org/10.13182/Nt09-A7403
Soderquist CZ, Johnsen AM, McNamara BK, Hanson BD, Chenault JW, Carson KJ, Peper SM (2011) Dissolution of irradiated commercial UO2 fuels in ammonium carbonate and hydrogen peroxide. Ind Eng Chem Res 50(4):1813–1818. https://doi.org/10.1021/ie101386n
Asanuma N, Harada M, Ikeda Y, Tomiyasu H (2001) New approach to the nuclear fuel reprocessing in non-acidic aqueous solutions. J Nucl Sci Technol 38(10):866–871. https://doi.org/10.3327/jnst.38.866
Peper SM, Brodnax LF, Field SE, Zehnder RA, Valdez SN, Runde WH (2004) Kinetic study of the oxidative dissolution of UO2 in aqueous carbonate media. Ind Eng Chem Res 43(26):8188–8193. https://doi.org/10.1021/ie049457y
Smith SC, Peper SM, Douglas M, Ziegelgruber KL, Finn EC (2009) Dissolution of uranium oxides under alkaline oxidizing conditions. J Radioanal Nucl Chem 282(2):617–621. https://doi.org/10.1007/s10967-009-0182-8
Stepanov SI, Boyarintsev AV, Chekmarev AM (2009) Physicochemical foundations of spent nuclear fuel leaching in carbonate solutions. Dokl Chem 427(2):202–206. https://doi.org/10.1134/S0012500809080060
Boyarintsev AV, Stepanov SI, Chekhlov AA, Chekmarev AM, Tsivadze AY (2016) Chemistry of the CARBEX process: identification of absorption bands of the ligands in the electronic spectra of aqueous solutions of Na4[UO2(O2)CO3)2]. Dokl Chem 469:227–232. https://doi.org/10.1134/S001250081607003X
Goff GS, Brodnax LF, Cisneros MR, Peper SM, Field SE, Scott BL, Runde WH (2008) First identification and thermodynamic characterization of the ternary U (VI) species, UO2(O2)(CO3)24−, in UO2−H2O2−K2CO3 solutions. Inorg Chem 47(6):1984–1990. https://doi.org/10.1021/ic701775g
Kim KW, Jung EC, Lee KY, Cho HR, Lee EH, Chung DY (2012) Evaluation of the behavior of uranium peroxocarbonate complexes in Na–U (VI)–CO3–OH–H2O2 solutions by Raman spectroscopy. J Phys Chem A 116(49):12024–12031. https://doi.org/10.1021/jp307062u
Kim KW, Kim YH, Lee SY, Lee JW, Joe KS, Lee EH, Kim JS, Song K, Song KC (2009) Precipitation characteristics of uranyl ions at different pHs depending on the presence of carbonate ions and hydrogen peroxide. Environ Sci Technol 43(7):2355–2361. https://doi.org/10.1021/es802951b
Kim KW, Hyun JT, Lee EH, Park GI, Lee KW, Yoo MJ, Song KC, Moon JK (2011) Recovery of uranium from (U, Gd)O2 nuclear fuel scrap using dissolution and precipitation in carbonate media. J Nucl Mater 418(1–3):93–97. https://doi.org/10.1016/j.jnucmat.2011.06.019
Zhu Z, Noël JJ, Shoesmith DW (2020) Hydrogen peroxide decomposition on simulated nuclear fuel bicarbonate/carbonate solutions. Electrochim Acta 340:135980. https://doi.org/10.1016/j.electacta.2020.135980
Nilsson S, Jonsson M (2011) H2O2 and radiation induced dissolution of UO2 and SIMFUEL pellets. J Nucl Mater 410(1–3):89–93. https://doi.org/10.1016/j.jnucmat.2011.01.020
Du Preez J, Morris D, Van Vuuren C, Oertell M (1980) The chemistry of uranium. Part XXVI. Alkaline dissolution of gold and/or uranium dioxide powders. Hydrometallurgy. 6(1–2):147–158
Pierce EM, Icenhower JP, Serne RJ, Catalano JG (2005) Experimental determination of UO2(cr) dissolution kinetics: effects of solution saturation state and pH. J Nucl Mater 345(2–3):206–218. https://doi.org/10.1016/j.jnucmat.2005.05.012
Smith SC, Peper SM, Douglas M, Ziegelgruber KL, Finn EC (2009) Dissolution of uranium (IV) oxide in solutions of ammonium carbonate and hydrogen peroxide. Pacific Northwest National Lab.(PNNL), Richland, WA (United States), United States
Chekmarev AM, Boyarintsev AV, Stepanov SI, Tsivadze AY (2017) Physicochemical principles of preparation of U (VI) carbonate solutions for extraction reprocessing in the Carbex process. Radiochemistry 59(4):345–350. https://doi.org/10.1134/S106636221704004X
Hou CX, He H, Sun JJ, Yang B, Fang HF, Jiao CS, He MJ (2022) Dissolution of uranium dioxide powder in carbonate-peroxide solution. J Radioanal Nucl Chem 331(5):2245–2252. https://doi.org/10.1007/s10967-022-08263-8
Hou CX, He MJ, Fang HF, Zhang M, Gao Y, Jiao CS, He H (2022) Ultrasonic-assisted dissolution of U3O8 in carbonate medium. Nucl Eng Technol. https://doi.org/10.1016/j.net.2022.09.025
Neese F (2011) The ORCA program system. WIREs Computational Molecular Science 2(1):73–78. https://doi.org/10.1002/wcms.81
Neese F (2017) Software update: the ORCA program system, version 4.0. WIREs Computational Molecular Science. https://doi.org/10.1002/wcms.1327
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110(13):6158–6170. https://doi.org/10.1063/1.2148954
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113(18):6378–6396
Schäfer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100(8):5829–5835. https://doi.org/10.1063/1.467146
Küchle W, Dolg M, Stoll H, Preuss H (1993) Energy-adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J Chem Phys 100(10):7535–7543. https://doi.org/10.1063/1.3382344
Cao X, Dolg M (2004) Segmented contraction scheme for small-core actinide pseudopotential basis sets. J Mol Struct 673(1–3):203–209. https://doi.org/10.1016/j.theochem.2003.12.015
Cao X, Dolg M, Stoll H (2003) Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials. J Chem Phys 118(2):487–496. https://doi.org/10.1063/1.1521431
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys 7(18):3297–3305. https://doi.org/10.1039/b508541a
Zheng J, Xu X, Truhlar DG (2010) Minimally augmented Karlsruhe basis sets. Theor Chem Acc 128(3):295–305. https://doi.org/10.1007/s00214-010-0846-z
Pantazis DA, Neese F (2011) All-electron scalar relativistic basis sets for the actinides. J Chem Theory Comput 7(3):677–684. https://doi.org/10.1021/ct100736b
Neese F, Wennmohs F, Hansen A, Becker U (2009) Efficient, approximate and parallel Hartree-Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree-Fock exchange. Chem Phys. 356(1–3):98–109. https://doi.org/10.1016/j.chemphys.2008.10.036
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Zanonato PL, Di Bernardo P, Szabó Z, Grenthe I (2012) Chemical equilibria in the uranyl (VI)–peroxide–carbonate system; identification of precursors for the formation of poly-peroxometallates. Dalton Trans 41(38):11635–11641. https://doi.org/10.1039/C2DT31282D
Watanabe T, Ikeda Y (2013) A study on identification of uranyl complexes in aqueous solutions containing carbonate ion and hydrogen peroxide. Energy Procedia 39:81–95. https://doi.org/10.1016/j.egypro.2013.07.194
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (U1967219)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, C., He, M., Li, C. et al. The oxidation dissolution of uranium oxides in carbonate-peroxide aqueous solution. J Radioanal Nucl Chem 332, 917–923 (2023). https://doi.org/10.1007/s10967-022-08652-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08652-z