Abstract
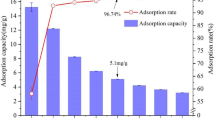
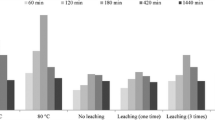
Efficient removal of 137Cs from radioactive wastewater and seawater environments is of great significance to environmental protection and human health. Therefore, it is urgent to develop an adsorbent with large adsorption capacity and high adsorption efficiency. However, the development of high-performance adsorbents depends on the understanding of their adsorption mechanisms. There is currently no systematic evidence for the mechanism by which sodium cupric ferrocyanide adsorbs cesium. In this paper, the adsorption mechanism of cesium was systematically studied by the synergistic analysis of ICP-MS, Raman, FT-IR, XRD, XPS, TGA and SEM using sodium cupric ferrocyanide prepared in the laboratory. We observed for the first time that after the adsorption of cesium ions by sodium cupric ferrocyanide, the cyano band of the Raman spectrum showed a distinct Cs-CN sub-peak. At the same time, the relative intensities of the Na-CN sub-peaks decreased significantly, while those of Fe-CN and Cu-CN barely changed. This result is consistent with the results shown by ICP-MS and confirms the fact that sodium ions exchange with cesium ions. The results of XRD, XPS, TG and FT-IR confirmed that the ion exchange did not cause structural damage, which indicated that the cesium adsorption process was a pure ion exchange. In addition, we also found that 0.493% of the total amount of cesium adsorbed may be due to the contribution of defect vacancies.








Similar content being viewed by others
References
Awual MR, Yaita T, Kobayashi T, Shiwaku H, Suzuki S (2020) Improving cesium removal to clean-up the contaminated water using modified conjugate material. J Environ Chem Eng 8(2):103684
Sk A, Mfc A, Mra B, Ai C, Tk D (2021) Efficient cesium encapsulation from contaminated water by cellulosic biomass based activated wood charcoal. Chemosphere 262:127801
Lee H, Choi S (2021) Copper ferrocyanide chemically immobilized onto a polyvinylidene fluoride hollow-fibre membrane surface for the removal of aqueous cesium. Environ Technol 43(15):2241–2251
Lee HK, Choi JW, Kim JH, Kim CR, Choi SJ (2021) Simultaneous selective removal of cesium and cobalt from water using calcium alginate-zinc ferrocyanide-Cyanex 272 composite beads. Environ Sci Pollut Res 28(31):42014–42023
Chen S, Hu J, Han S, Guo Y, Deng T (2020) A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade. Sep Purif Technol 251:117340
Avramenko V, SvetlanaBratskaya VeniaminZheleznov, IrinaSheveleva VO, Sergienko V (2011) Colloid stable sorbents for cesium removal: Preparation and application of latex particles functionalized with transition metals ferrocyanides. J Hazard Mater 186:1343–1350
Cattermull J, Pasta M, Goodwin AL (2021) Structural complexity in Prussian blue analogues. Mater Horizons 8(12):3178-3186
Azhar A, Li Y, Cai Z, Zakaria MB, Masud MK, Hossain M, Kim J, Zhang W, Na J, Yamauchi Y (2019) Nanoarchitectonics: a new materials horizon for prussian blue and its analogues. Bull Chem Soc Jpn 92:875–904
Han F, Zhang GH, Gu P (2013) Adsorption kinetics and equilibrium modeling of cesium on copper ferrocyanide. J Radioanal Nuclear Chem 295(1):369–377
Loos-Neskovic C, Ayrault S, Badillo V, Jimenez B, Garnier E, Fedoroff M, Jones DJ, Merinov B (2004) Structure of copperpotassium hexacyanoferrate (II) and sorption mechanisms of cesium. J Solid State Chem 177:1817–1828
Chen X, Li Y, Zhu L, Ke Y, Yang Y (2020) High-efficiency continuous enrichment of cesium ions using CuFC composite microspheres: dynamic adsorption and mechanism analysis. J Radioanal Nucl Chem 326:1–15
Lee I, Park CW, Yoon SS, Yang HM (2019) Facile synthesis of copper ferrocyanide-embedded magnetic hydrogel beads for the enhanced removal of cesium from water. Chemosphere 224:776–785
Delchet, Carole, Grandjean, Agnes, Barre, Yves, Larionova, Joulia, Causse, Jeremy (2016) Effect of the chemical nature of different transition metal ferrocyanides to entrap Cs. J Radioanal Nucl Chem Int J Deal Aspect Appl Nucl Chem 307(1):427–436
Cliffe MJ, Keyzer EN, Bond AD, Astle MA, Grey CP (2020) The structures of ordered defects in thiocyanate analogues of Prussian Blue. Chem Sci 11(17):4430–4438
Xiang S, Zhang X, Tao Q, Dai Y (2019) Adsorption of cesium on mesoporous SBA-15 material containing embedded copper hexacyanoferrate. J Radioanal Nucl Chem 320(3):609–619
Lee KY, Kim J, Oh M, Lee EH, Kim KW, Chung DY, Moon JK (2017) Effect on Cs removal of solid-phase metal oxidation in metal ferrocyanides. Radiochimica Acta 105(12):1043–1048
Liu J, Li X, Rykov AI, Fan Q, Xu W, Cong W, Jin C, Tang H, Zhu K, Ganeshraja AS (2017) Zinc-modulated Fe–Co Prussian blue analogues with well-controlled morphologies for the efficient sorption of cesium. J Mater Chem A 5:3284–3292
Ma C, Jiang Z, Han S, Guo Y, Deng T (2021) Novel one-pot solvothermal synthesis of high-performance copper hexacyanoferrate for Cs+ Removal from Wastewater. Hindawi Limited 9:3762917
Cho E, Lee J, Lee B, Lee K, Yeom B, Lee T (2020) Cesium ion-exchange resin using sodium dodecylbenzenesulfonate for binding to Prussian blue. Chemosphere 244:125589
Yang HM, Hwang KS, Park CW, Lee KW (2017) Sodium-copper hexacyanoferrate-functionalized magnetic nanoclusters for the highly efficient magnetic removal of radioactive caesium from seawater. Water Res 125:81–90
Zhu LK, Yao YY, Chen DZ, Lan P (2022) The effective removal of Pb2+ by activated carbon fibers modified by l-cysteine: exploration of kinetics, thermodynamics and mechanism. RSC Adv 12:20062–20073
Hasan MN, Shenashen MA, Hasan MM, Znad H, Awual, (2021) Assessing of cesium removal from wastewater using functionalized wood cellulosic adsorbent. Chemosphere 270:128668
Takahashi A, Tanaka H, Minami K, Noda K, Ishizaki M, Kurihara M, Ogawa H, Kawamoto T (2019) unveiling cs-adsorption mechanism of prussian blue analogs: cs + -percolation via vacancies to complete dehydrated state 8:34808
Nordstrand J, Toledo-Carrillo E, Kloo L, Dutta J (2022) Sodium to cesium ions: a general ladder mechanism of ion diffusion in prussian blue analogs. Phys Chem Chem Phys 24:12374
Wang X, Pandey S, Fullarton M, Phillpot SR (2021) Study of incorporating cesium into copper hexacyanoferrate by density functional theory calculations. J Phys Chem C Nanomater Interfaces 125(43):24273–24283
Ivanets AI, Shashkova IL, Drozdova NV, Davydov DY, Radkevich AV (2014) Recovery of cesium ions from aqueous solutions with composite sorbents based on tripolite and copper(II) and nickel(II) ferrocyanides. Radiochemistry 56:524–528
Oh D, Kim B, Kang S, Kim Y, Hwang Y (2019) Enhanced immobilization of Prussian blue through hydrogel formation by polymerization of acrylic acid for radioactive cesium adsorption. Sci Rep 9:16334
Akemoto Y, Sakti S, Kan M, Tanaka S (2021) Interpretation of the interaction between cesium ion and some clay minerals based on their structural features 28(11):14121–14130
Wang J, Zhuang S (2019) Removal of cesium ions from aqueous solutions using various separation technologies. Rev Environ Sci Bio/Technol 18(2):231–269
Kim M, Lee W, Kim] S (2018) Rapid removal of radioactive cesium by polyacrylonitrile nanofibers containing Prussian blue. J Hazardous Mater 347:106–113
Liu X, Chen GR, Lee DJ, Kawamoto T, Tanaka H, Chen ML, Luo YK (2014) Adsorption removal of cesium from drinking waters: a mini review on use of biosorbents and other adsorbents. Biores Technol 160:142–149
Kim Y, Eom HH, Kim D, Harbottle D, Lee JW (2021) Adsorptive removal of cesium by electrospun nanofibers embedded with potassium copper hexacyanoferrate. Sep Purif Technol 255:117745
Wi H, Kim H, Oh D, Bae S, Hwang Y (2019) Surface modification of poly(vinyl alcohol) sponge by acrylic acid to immobilize Prussian blue for selective adsorption of aqueous cesium. Chemosphere 226:173–182
Le L, Nguyen SA, Nguyen TD, Le V, Cao HV, Nguyen NB, Le T (2021) Prussian Blue Analogues of A2[Fe(CN)6] (A: Cu2+, Co2+, and Ni2+) and Their Composition-Dependent Sorption Performances towards Cs+, Sr2+, and Co2+. J Nanomater 12:5533620
Xta B, Sw A, Zz C, Zl A, Lin WA, Ly A, Bw D, Jian SD, Lz E, Hw B (2022) Graphene oxide/chitosan/potassium copper hexacyanoferrate(II) composite aerogel for efficient removal of cesium. Chem Eng J 444:136397
Ke Y, Li Y, Zhu L, Zhou Y, Liu D (2020) Rapid enrichment of cesium ions in aqueous solution by copper ferrocyanide powder. SN Appl Sci 2:522
Mihara Y, Sikder MT, Yamagishi H, Sasaki T, Kurasaki M, Itoh S, Tanaka S (2016) Adsorption kinetic model of alginate gel beads synthesized micro particle-prussian blue to remove cesium ions from water. J Water Process Eng 10:9–19
Yk A, Hhe A, Yun K, Dh B, Jwl A (2020) Effective removal of cesium from wastewater via adsorptive filtration with potassium copper hexacyanoferrate-immobilized and polyethyleneimine-grafted graphene oxide. Chemosphere 250:126262
Huo J, Yu G, Wang J (2020) Selective adsorption of cesium (I) from water by Prussian blue analogues anchored on 3D reduced graphene oxide aerogel. Sci Total Environ 761:143286
Lee, Hyun-Kyu, Choi, Jung, Wonzin, Sang-June. (2016) Sorption of cesium ions from aqueous solutions by multi-walled carbon nanotubes functionalized with copper ferrocyanide. J Radioanal Nucl Chem 309(2):477–484
Li X, Wu X, Chen J, Li Y, Yang Y (2019) Alginate-enfolded copper hexacyanoferrate graphene oxide granules for adsorption of low-concentration cesium ions from aquatic environment. J Radioanal Nucl Chem 320:655–663
Ivanets A, Milyutin V, Shashkova I, Kitikova N, Nekrasova N, Radkevich A (2020) Sorption of stable and radioactive Cs(I), Sr(II), Co(II) ions on Ti–Ca–Mg phosphates. J Radioanal Nucl Chem 324:1115–1123
Acknowledgements
This work was supported by the Zhejiang Province Public Welfare Project (NO. LGG20E030009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, L., Chen, C., Hou, H. et al. Structure analysis and cesium adsorption mechanism evaluation of sodium copper ferrocyanide. J Radioanal Nucl Chem 331, 5835–5842 (2022). https://doi.org/10.1007/s10967-022-08633-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08633-2