Abstract
Ferruginous sandstone considered as a one of the economically significant uranium mineralized areas in the southwestern part of the Sinai that located at the lower member of Um Bogma Formation in Abu Thor area. The studied sample was characterized geologically as a sandstone rock contain 450 mg/kg of uranium. This work is aimed to compare between eco-friendly bioleaching and conventional leaching processe. The uranium bioleaching efficiency achieved 80% by using a native fungal strain; Aspergillus nidulans as a green technology with the optimimum conditions as; 3 pH value, 3% pulp density, 7 days incubation period, and 30 °C incubation temperatures. Whereas the traditional technique using alkaline and sulphuric acid leaching attained 68.5% and 95% respectively at the best condition. Finally, the shrinking core model was represented the layer diffusion process by acquiring apparent activation energy 18.28 and 16.82 kJ/mol for carbonate and sulphuric acid leaching, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term "conventional resources" refers to resources that can be recovered as a primary product, a co-product or an important by-product. Unconventional resources, on the other hand, are those from which can only be recovered as a minor by-product. Uranium is associated with phosphate rocks, non-ferrous ores, carbonatite (carbonate), black shale and lignite. Most of the unconventional uranium resources reported to date are associated with uranium in black shale and phosphate rocks [1]. Phosphate rocks are considered as one among the most popular and valuble resources of uranium according to the relatively large concentration of accompanying uranium as a result of its abundance and the large global trade volumes of phosphate rocks, when phosphate rocks are digested by acid as H2SO4 in the process of phosphoric acid production, significant amounts of phosphogypsum were obtained. This phosphogypsum has low levels of radioactivity that permit to use in the plants processing, whereas the radioactivity 80–90% involved in the phosphoric acid stream [2]. Also, granites rocks from Gabal El-Missikat are one of the worthiest of attention appearance of uranium Central Eastern Desert, Egypt. Uranium content ranging from 497 to 8845 mg/kg has been recorded in the shear zone at El Missikat granitic pluton [3]. Furthermore, high contents of radioactive minerals were noticed in the Egyption black sand [4].
Uranium leaching is applied using conventional or non-conventional techniques. The traditional leaching comprises several techniques as acidic, alkaline, autoclave pressure, strong acid pug, and curing leaching. The choice of traditional leaching using chemical as acidic or alkaline lixiviant is encouraged by mineralogy of the host rock was stated according to Youltona and Kinnaird [5]. In alkaline leaching using solution of Na2CO3 and NaHCO3 as lixiviant in the recovery of uranium from the ores hosted in alkaline rocks such as carbonates (> 15%) [6]. The alkaline leaching technique is generally characterized by producing a comparatively pure solution due to its relative selectivity. Consequently, it is used as an easy regeneration and recycling process in addition to its lower corrosively property and low waste discharge [7]. Acid leaching refers to the use of H2SO4 as lixivant in all common practice. HNO3 has strong oxidizing properties but is much more expensive than H2SO4 and will form uranyl nitrate in dilute solutions which are incompatible with the use of anion type ion exchange, it was used at one time to treat very high-grade ores which are not now available. HCl is used for employing the salt roasting process, since the acid is recovered from the roaster gases; otherwise, hydrochloric acid is too expensive for regular use and has the added disadvantage of being highly corrosive. Several authors studied the acidic and alkaline uranium leaching from different ores [8,9,10,11,12].
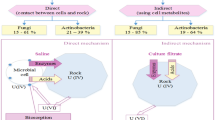
The environment encloses mix of numerous components including all of the physical and biological surrounds, as well as the interactivity of living and nonliving things that take place naturally on Earth through chemical and physical phenomena could help to understand the living species interactions and improve the state of the environment's health. Biotechnology encompasses the entire scientific process as well as the principles of engineering. Environmental biotechnology is regarded as an engine for integrated environmental protection, leading to sustainable development [13]. Every day the potential risks of toxic chemical concentrations are increasing in the environment, cause considerable disadvantages of the surrounding environment. Bioaccumulation occurs although some pollutants present in very small quantities within the environment, where a living organism can take up and concentrated it, and by time incorporating trophic genera and biomagnifying specific toxicity. Biotechnology enhanced the environmental possibilities of pollution prevention, solid waste, and wastewater treatments, manufacturing with less pollution or less raw materials, and to eliminating environmental hazards and risks in the form of biotreatment or bioremediation [13]. Recently, bioleaching is a novel used technology; it is a natural, simple, and effective interaction between microbes and minerals, also recognized as a technology that is used to extracts the valuable metals from a low-grade ore with the help of microorganisms as bacteria or fungi. The biotechnology was more effective and more responsive to the environment than others. The extraction of essential minerals from low-grade ores becomes inevitable. Traditional extraction through chemical leaching is less profitable compared to bioleaching. The dissolution process of metals from their minerals naturally by the occurrence of certain microorganisms defined as bioleaching process, at the same time can be taken out from any matter when the solution is filtered through it [14]. Particularly, bioleaching refers to the diversion of insoluble valuable metals into their soluble phase by using of microorganisms that play a catalytic role, and It is essential to industrialize the recovery of heavy metals by biological leaching, which produce non-harmful gases to form secondary pollution during the entire reaction process. Consequently, by comparing to other methods such as chemical leaching, bioleaching was found to better recover valuable metals from low concentrations whose extraction was not economically feasible with the former [15, 16]. Bioleaching profiles of mineral samples from each mineralization are unique because of variation in inclusion and mineral composition, acid demand characteristics, and concentration of uranium. Uranium could be recovered by microorganisms that catalyze the oxidation and reduction of uranium and their associated metals. Amin et al. [14] applied the bioleaching technique for uraniferrous sedimentary rocks from southwestern Sinai, Egypt, using two native isolated strains Aspergillus niger and Aspergillus terreus, which exhibited the highest leaching efficiencies of uranium. The recovery of uranium from low-grade ore using Aspergillus niger isolated from uraniferrous rock sample accomplished 71.4% and asserted that the uranium is nearly free from other radionuclides present in the original sample without any subsequent purification processes, whereas could not achieved by the conventional process of uranium recovery [17]. Abhilash et al. approximately extracted 70% of uranium from Turamdih ore, India at 1.7 pH in 60 days time with average particles of 10–12 mm size using A. ferrooxidans [18].
This work aims to compare between the bio and chemical techniques in uranium leaching that achieved maximum uranium leaching efficiency with lower cost, which applied upon a low-grade sandstone sample collected from Abu Thor area in depth four meters.
Geological aspects
Abu Thor area is located in the southwestern part of Sinai Peninsula Fig. 1a. It represents one of the more considerable uranium mineralized areas wherever, it was described to various detailed geological, geophysical, structural, geochemical, mineralogical, and radiometric studies. Um Bogma Formation is characterized by uranium mineralization, which associated well and developed in Abu Thor area at thickness ranging from 5 to 12 m. The studied area is located between 33° 21 ˋ—33° 23ˋ E and 29°—29° 05ˋ N Fig. 1b is considered a part of east Abu Zeneima promising area. The Paleozoic outcrops of southwestern Sinai were subdivided and classified into series as follow: (i) Lower Sandstone Series, (ii) Carboniferous Limestone Series, and (iii) Upper Sandstone Series [19]. The studied area was demonstrated in Table 1 that known as the Paleozoic lithostratigraphic section. The first geological map was compiled for west central Sinai area and assigned the Upper Sandstone Series from the early to late Carboniferous age. Then the Um Bogma Formation term was applied to the Carboniferous Limestone Series [20]. The Lower Sandstone sequence was classified into several formations that called as Sarabit El Khadim, Abu Hamata, and Adedia formations. On the other hand, Abu Zarab, Magharet El-Maiah, and El-Hashash formations were subdivided from the Upper Sandstone sequence [21], whereas the Abu Thora Formation term was introduced instead of the upper sandstone sequence expression [20, 22]. The basaltic sheet capping the Paleozoic rocks has 60 m thickness was concluded by Hussein et al. [23] and was noted by Said [24] as Triassic age. Finally, the studied area showed high eU content found with some facies as; ferruginous siltstone, ferruginous sandstone, shale, carbonaceous shale, marl, gibbsite bearing sediments, sandy dolostone, and Fe–Mn ore of Um Bogma Formation Fig. 2a, b, c.
Experimental
Ore characterization and chemical analyses
The studied sample was collected from Abu Thor that is located in southwestern Sinai, Egypt. The sample has been grinding to -200 mesh (≈ 75 μm), and quartering to obtain a representative sample. Complete chemical analysis of both major and trace elements was performed on about 5 g from the sample. The major element oxides were analyzed using the rapid silicate analytical procedure [25] with ± 2 wt.% error for most oxides. On the other hand, the loss on ignition (L.O.I) was gravimetrically determined at 110 °C, 550 °C and 1000 °C for H2O−, organic matter and CO2, respectively. While the trace elements had been characterized by the X-ray fluorescence technique (XRF), (Philips Unique II unit fitted with an automatic sample changer PW 1510, 30 position), which connected to a computer system using X-40 program for spectrometry in the Nuclear Materials Authority Laboratories (NMA Lab).
Furthermore, determination of uranium was exhibited by an oxidimetric titration method using ammonium metavanadate that was used after its reduction in the presence of diphenyl amine-4-sulfonic acid sodium salt as indicator [26]. The titration was repeated at least three times for each sample and the experimental error was less than 3%. Uranium can be calculated according to Eq. 1
where, T is the titration intensity of NH4VO4 [Ammonium meta-Vandate] solution, V1 is the volume of NH4VO4 solution consumed and V is the volume of the measured sample.
Chemical leaching procedures
The leaching process is highly dependent upon number of factors that must be carefully considered to obtain the best conditions, for that, several leaching experiments had been performed using alkaline and acidic reagents. The studied factors involved alkali or acid concentrations, agitation leaching time, temperature, and liquid to solid ratio. For this purpose, the leaching experiments were carried out upon 10 g of an ore sample. After each experiment, filtrate the leach slurry, fully washed with distilled water, both filtrate and washing were made up to volume (50 ml) before analysis. All leaching experiments were done in triplicate, and only the average values were reported. The maximum errors were less than 3%. Finally, the leachability of uranium was evaluated according to Eq. 2
Bioleaching techniques
The microbial studies involve the isolation from the sample and the conditioning of the leaching factors as follows:
Preparation of media
The agar media g/ l was prepared as follows: 40 g/l dextrose as a acarbon source, 10 g/ l peptone, and 20 g/l agar. The pH value of the medium was adjusted to reach 5.6. The bioleach liquor preparation was 100 ml of broth solution placed in 250 ml Erlenmeyer flask, and then the flask was complemented with 1 g. of an ore sample. The flasks were autoclaved at 120 °C for 20 minutes under pressure equals 1.5 atm. and were inoculated with 1 ml of spore suspension after cooling, and then incubated at 30 °C for 7 days. Finally, the culture medium was filtered several times and also, the filtrate was utilized for chemical determination. The direct plating technique was utilized in fungal isolation from the sample, which spread fine ore powder directly upon the surface of solid agar media under sanitize conditions. Incubation of the agar plates were at 28 °C ± 2 until the fungal colonies clearly turned up, then the purified fungi were identified morphologically [14].
Factors affecting uranium bioleaching
Various factors were studied with the aim of acquiring the bioleaching optimum conditions of uranium as; initial pH values, pulp densities, finally, incubation periods and temperatures. 100 ml of broth was placed in the flasks, then complemented with sample concentrations. The autoclaved flasks were inoculated with 1 ml of spore suspension. Finally, filtrate the culture medium and centrifuged at 4000 rpm, repeated for each factor. The filtrate was utilized for uranium determination.
Results and discussion
Chemical characteristics of sample
From the obtained data of the major constituents of the studied sample Table 2, it is clearly evident that the host rock is mainly involved of SiO2, Al2O3, Fe2O3, CaO and MgO assaying 33.71, 17.8, 16.4, 8.81, and 2.62%, respectively. Besides that, it assays 3035, 628, 487, 470, and 450 ppm for Cu, V, Zn, Co, and U respectively.
Chemical alkaline leaching
Different alkaline reagents types
In the present work various leaching agents were examined upon the uranium leaching efficiency, using 100 g/l leaching agents as a concentration, keeping other leaching conditions fixed at 85 °C leaching temperature, for 120 min leaching time and (3/1) liquid/solid. The obtained data was demonstrated in Table 3, which indicated that the preferable reagent was a (3:1) of Na2CO3 and NaHCO3 mixture from a total concentration of 100 g/l which exhibited 42.11% leaching efficiency of uranium. The chemistry of uranium leaching ores with Na2CO3 and NaHCO3 reagent combine was explained by the following chemical reaction steps Eqs. 3, 4, which achieved by oxidation of UIV to UVI by a suitable oxidant [27].
From the above second reaction Eq. 4, the excess of sodium hydroxide that produced would increase the pH of the solution; a matter which would result in uranium precipitation. In order to prevent such an increase in the pH of the solution, sodium bicarbonate is generally used to buffer the hydroxide ions [27] Eq. 5. The following reaction Eq. 6 can thus represent the overall reaction
Effect of different concentrations of mixed alkali
The effect of alkaline mixed concentration ranging start with 50 up to 200 g/l upon uranium leachability, leaving the other conditions fixed at 85 °C leaching temperature for 120 min leaching time and (3/1) liquid/solid ratio. As shown in Fig. 3, the uranium leaching efficiency was increased from 32.2 to 68.5% by increasing the leaching reagent concentrations from 50 to 150 g/l, and then dramatically decrease in uranium leaching efficiency was observed, which recorded 49.78% at 200 g/l reagent concentration, the presence of some gangue minerals such as silica and alumina which commonly form the main ore host in the studied sample assaying 33.71% and 17.8%, respectively may be lead to consume leaching reagent according to the following equations Eqs. 7, 8 [28].
which lead to decrease uranium leaching efficiency, for that 150 g/l of mixed (3:1) Na2CO3/ NaHCO3 considered as preferred conditions for other leaching experiments.
Effect of H2O2 concentration
To determining the effect of H2O2 upon the uranium leachability; examined the unavailability and the existence of H2O2 with several concentrations varying from 0.5 to 2.5 M with stationary leaching conditions were at 150 g/l as a concentration of mixed alkali, liquid/solid ratio of 3/1 for 120 min leaching time, and at 85 °C leaching temperature. Figure 4 indicated that the absence of oxidant effect at trial to improve the leachability, this is due to the presence of uranium in the studied ore sample as a secondary mineral in the form of UVI instead of any other state of oxidation, particularly UIV, which is in agreement with explanation of Desouky et al. [29].
Effect of contact time
The contact time of uranium leaching was altered from 30 to 210 min, while the other leaching conditions were fixed at 150 g/l of alkaline mixed concentration with 3/1 as a liquid/solid ratio at leaching temperature 85 °C. From data obtained in Fig. 5, the leaching efficiency of uranium increased with increasing the contact time accomplished 68.5% at 120 min. Otherwise, the uranium leaching efficiency was decreased by increasing the leaching contact time, consequently, 120 min was chosen as the best contact time.
Effect of mixed alkali liquid/solid ratio
To study the effect of mixed alkaline liquid/solid ratio upon uranium leachability at the concentration of 150 g/l mixed (3:1) Na2CO3/ NaHCO3, several experiments were carried out from 1/1 to 5/1 at 85 °C leaching temperature and 120 min leaching contact time. Figure 6 displayed that the leaching efficiency of uranium was increased with increasing the ratio of mixed alkali to reach 68.50% at a 3/1 liquid/solid ratio. Furthermore, the results of uranium leaching efficiency were decreased to reach 54.0% by increasing in the liquid/ solid ratio to 4/1 and 5/1, that can be explained by the dissolution of the interfering elements [11]. Hence L/S ratio of 3/1 was considered as the best condition for other leaching experiments.
Effect of temperature
The factor of temperature affect uranium leachability was studied from the scale of the room temperature raising to 85 °C, the remaining conditions have been fixed at 150 g/l of mixed alkali concentration (3:1) of Na2CO3/ NaHCO3 and 3/1 as liquid/solid ratio (L/S) for 120 min leaching contact time. Figure 7 indicated the leachability of uranium was increased from 35.56 to 68.5% via increasing of the temperature scale from 25 to 85 °C, respectively. Thus, the preferred temperature for the alkaline leaching process is 85 °C.
From the previous results of the alkaline leaching process, the studied factors of the working sample, it can be concluded the optimum leaching condition was occurring at 150 g/l mixed alkaline solution of (3:1) Na2CO3/ NaHCO3 concentration, 120 min leaching contact time, 3/1 liquid/ solid ratio at 85 °C, which achieved 68.5% of leaching efficiency uranium.
Chemical acid leaching
The leaching with sulfuric acid is the predominant recovery process of uranium from rocks. The efficiencies of leaching in sulfuric acid reach 85–95%. However, this method is not appropriate for the leaching of uranium from carbonate rocks due to high acid consumption. It is worth to note that the alkaline leaching is more selective for uranium in comparison with acid processing [12]. In this study, the maximum leaching efficiency of uranium using alkaline leaching was not increased than 68.5%. Despite the highest concentration of carbonates (16.27% L.O.I., 8.81% CaO and 2.62% MgO) Table 2, the decision to investigate the effect of acid reagent upon uranium leachability should achieve a high leachability.
Effect of acid type
The acid type as a factor affects upon uranium leaching efficiency for the studied ore sample had been investigated with sulphuric, hydrochloric, and nitric by using 150 g/l of each under these subsequent conditions 3/1 liquid/solid ratio for 180 min at room temperature. Table 4 demonstrated the high leachability of uranium, which reached 40% by applying H2SO4 comparing with other acids; therefore, it is decided to use sulphuric acid for other experiments.
Effect of H2SO4 concentration
There are different forms of uranium are represented as ions in acidic sulfuric acid solutions, such as UO22+, UO2SO4, [UO2(SO4)2]2−, and [UO2 (SO4)3]4− [30]. Leaching of UVI with sulphuric acid results in the formation of various uranyl sulphate complexes in solution as following Eqs. 9–11:
A sequence of experiments utilizing different H2SO4 concentrations beginning of 50 to 300 g/l was studied under the else fixed factors of (3/1) liquid /solid ratio, 180 min, and 25 °C. Fig. (3) clarified the increasing of uranium leachability from 26.0 to 64.0% by increasing the H2SO4 concentration from 50 to 200 g/l, further increase in acid concentrations the leaching efficiency remained almost unchanged indicating that the working conditions are not adequate; therefore, 200 g/l H2SO4 was chosen as best concentration applied in the subsequent experiments.
Effect of acid contact time
This factor was carried out by gradually change the leaching contact time starting with 60 to 300 min at 200 g/l of acid with (3/1) L/S ratio at room temperature. As shown in Fig. 5 the leachability of uranium was achieved an increasing percentage initiated from 46.5 to 64.0% by increasing the contact time to180 min, then after that time lead to a dramatic plateau in uranium leaching efficiency, the presence of calcium and magnesium compounds may be reacted with sulfuric acid easily by forming CaSO4 and MgSO4, where they possess low solubility and reprecipitated or adhered to the uranium particles surface, generating block of tiny channels and micropores causing an immersion dead angle (leaching blind area). Also, the leaching solution can not be able to migrate and diffuse into the inner of the ore particles, consequently, the leaching rate decreased in the ore sample [31] therefore, 180 min. was used as the best leaching agitation contact time.
Effect of liquid/solid ratio
The effect of the liquid/solid ratio was examined from 1/1 to 4/1. These experiments occurred at 200 g/l acid concentration, 180 min agitation contact time, and at room temperature. From the acquired results Fig. 6, an increase of the uranium leachability was observed with the increase in the liquid/ solid ratio reached 64% at (3/1) liquid/solid ratio but by increasing the liquid/ solid ratio the decrease of uranium leachability was ascertained. For that, L/S ratio of 3/1 was considered as the best condition.
Effect of temperature
The temperature as a leaching factor was increased in the range from the room (about 25 °C) up to 100 °C was studied at 200 g/l of acid and (3/1) liquid/solid ratio for 180 min agitation contact time. Increasing temperature from 25 to 85 °C leading to an increase in the leaching efficiency of uranium from 64.0 to 95.0% and recorded no effect on uranium leaching efficiency by increasing temperature Fig. 7, for that 85 °C was chosen as the best leaching temperature.
Finally, from the above results of the acid agitation leaching concluded that the acid leaching was better than alkaline leaching technique with 95% uranium leaching efficiency, at the best conditions of 200 g/l H2SO4 concentration with a (3/1) liquid/solid ratio for 180 min leaching contact time and 85 °C leaching temperature. The acid method becomes unprofitable as the carbonate content increases due to increased acid consumption.
Kinetics of uranium chemical leaching
The kinetics of heterogeneous leaching reactions like leaching of minerals from its ores can be distinguished by the non reacted shrinking core model. In this model, the rate of reaction is under control by the following: liquid- film diffusion, product layer diffusion and surface chemical reaction, but due to the effect of stirring, the liquid –film diffusion was eliminated. The integration rates expressions for both product layer diffusion control and chemical control are given in Eqs. 12, 13 [32] as the following:
where kd and kc are the apparent rate constant (min−1) of diffusion through the product layer and surface chemical reaction, t is the reaction time and X is the reacted uranium ore fraction was expressed in Eq. 14 as the following:
The reaction mechanism is determined by the degree of linearity of the plot of the two previous equations against the time (t). For that, some reaction models were investigated to find which kinetic equation can fit the reaction isotherms. The effect of the temperature upon the uranium leaching reaction rate in the range of 298–358 K under fixed conditions for alkaline leaching as 150 g/l of mixed (3:1) Na2CO3/ NaHCO3 and (3/1) liquid/solid ratio from 30 to 120 min leaching contact time whereas under the following conditions for acidic leaching as; 200 g/l of H2SO4, (3/1) liquid/solid ratio, and from 60 to 180 min leaching contact time. Figures 8, 9 explained that the leaching efficiency of uranium was increased with the temperature increasing.
There are two shrinking core models had been examined at different temperatures as shown in Figs. 10, 11 for uranium leaching using alkali and acid Figs. 12, 13. It was found that the data did not fit with Eq. 13, while the best fit was achieved with Eq. 12, which be realized a straight line passing through the origin. On the other hand, Tables 5, 6 summarized the different values of the apparent rate constant (k) at different temperatures and their corresponding rates of the correlation coefficient for uranium leaching utilizing alkali and acid respectively. The better linearity at the value of R2 is closer to1.0. From the obtained data Table 5. of the alkaline leaching, the kd values yielded a change in the range of 0.0004–0.002 min−1 and R2 range between 0.9584 and 0.9941, while for kc in the range of 0.0013–0.003 and R2 were between 0.7915 and 0.9012. Furthermore, for the acidic leaching Table 6. summarized the kd constant values in the range of 0.0011–0.0037 min−1 and values of R2 were in the range of 0.9664–0.9972, for kc values exhibited variation in the range of 0.0018–0.0037 min−1, and R2 in the range of 0.8118–0.9585. Depending on the R2 values, the model of chemical reaction control is smaller than those for the product layer diffusion control at various temperatures. For that, it can be concluded the linear correlation between [1 – 3 (1-X).2/3 + 2 (1-X)] and leaching contact time (t) is suggested that the mechanism of leaching uranium is diffusion controlled through the product layer for both alkaline and acid leaching. This result is also confirmed by evaluating the activation energy of the uranium leaching reaction which may be expressed by Arrhenius equation Eq. 15
where, k, A, Ea, R, expresses the rate of reaction constant (min−1), the frequency factor (min−1), the apparent activation energy, the universal gas constant, and T is an absolute temperature (Kelvin). According to Arrhenius equation, the relation between the logarithm of the rate of reaction [ln (k)] and inverse temperature (1/T) will plot a straight line. The slope of this straight line presented the value of –Ea/R Fig. 14. The evaluated values of the activation energy were 18.28 kJ/mol and 16.82 kJ ∕ mol for alkali and acid leaching respectively, and this is consistent with Crundwell [33], who concluded that the value of the activation energy is lower than 20 kJ/mol for diffusion-controlled reactions and higher than 40 kJ/mol for chemical controlled reactions [33].
Bioleaching process
Fungal solubilization of metals is attained through bioleaching reaction mechanisms as; acidolysis (acid–base reaction), complexolysis / chelation formation, redoxolysis, and bioaccumulation [34]. The mechanism of acidolysis is dependent upon the in direct protonation process which is made by the fugal production of metabolites like enzymes, organic acids, and proton-translocating ATPase of the plasma membrane considered as an origin of H+ etc., causing the protonation of oxygen atoms of the sample metal oxides through reduction reaction Fig. 15 [35]. The importance role of the organics acids, which secreated by the fungi are: (i) to maintain the required pH for bioleaching process and improve it, (ii) to minimize the access of anions to the cations in a metal compounds reaction, thereby increasing the metal ions solubility, and (iii) to produce the hydrogen ions (H+), which enhance the mobilization of metal ions while stabilizing the metal chelation. On the other hand, fungi release amino acids, which play an important role in complexolysis process Fig. 16 [35]. Complexolysis mechanism is slower than acidolysis one. The formation of metal complexing ligands with other metabolites as low molecular weight chelating agent secreted by the fungi, however metal ions solubilization is based on the capacity of molecules to form complex metal chelating agents with strong bonds than the lattice bonding between metal ions with solid particles, enhancing the bioleaching process. The metal complexes stability in the solutions minimizes the toxic effect of metal ions on the fungi. The complexion process may reduce the toxicity of fungal metals when elevated levels of metals are present [36]. Furthermore, another mechanism known as redoxolysis (oxide reduction reaction) can be carried out during the bioleaching process. It depends on the type of the metal that used in the leaching process and its oxidation state, thus facilitating fungal leaching. The metals can be solubilized by enzymatic reactions activity in a direct method of the leaching process by an electron transfer through a physical contact between the fungi and the sample, causing the destruction of the mineral [34]. In the method of direct leaching of metals from a solid structure can occur across.; (i) oxidation or reduction reactions implies the electron transfers also from the solid structure (oxidation) to an electron acceptor (normally oxygen), or (ii) the electrons flow (reduction) from electron donor into the solid structure. The maximum efficiency of uranium can be achieved only when the bioleaching conditions correspond to the optimum growth conditions of the microorganisms. Unlike the previously mentioned mechanisms, bioaccumulation is the only mechanism that independent of the fungal excretion of metabolites, but through the fungal cell wall, which has a number of different functional groups as active sites which can link metallic ions (e.g., hydroxyl, amine, carboxyl, phosphate and sulfate groups) [37].
The isolated native fungal strain from the studied sample, which has a long-time soil construction (heavy metals) exposure can lead to physiological adaptation or considerable modification of their microbial populations affecting their activity, such changes maybe associated with alteration in the fungal capacity and their behaviour to interact with metals. It was identified morphologically belongs an Aspergillus species known as Aspergillus nidulans (A. nidulans) which was used in the bioprocess in the present work.
The effectiveness of uranium bioleaching capacity depends upon several parameters as: the properties of the microorganisms, ore /mineral type (surface area of the minerals, sample particle size, water availability), temperature, pH, redox potential, oxygen and carbon dioxide supply, supply of other nutrients (nitrogen compounds and phosphate) and toxic substances, and formation of secondary minerals. Consequently, this study is concerned with the determination of the major parameters of the bioleaching controlling factors as follows:
Initial pH
The pH is considered as a significant factor affects the fungal activity to metal leaching. It is important to optimize the initial pH to provide suitable pH to maintain its growth and activity. An acidic media may be maintained in order to keep uranium in solution facilitate leaching process. Acidity is controlled by the oxidation of the sample metal ions (iron, sulfur, metal sulfides) by the hydrolysis process (of ferric iron as an example). So, pH value of the leach liquor should be optimal for providing the maximum growth of the microorganisms, as well as supply the maximum solubilization efficiency of metals. The final pHs decreased with increasing the initial pH values of the growth medium. The optimum pH value was observed equals 3, it is apparently the best for the uranium bioleaching efficiency, which reached 86%. The optimum pH value was determined by utilizing the fungal strain A. nidulans at room temperature for 7 days on a peptone broth media recorded the highest leachability at 1% of the studied sample. Figure 17 showed that the amount of solubilized uranium decreased with increasing the initial pH value of the growth media. The type of primary metabolites produced by fungi and the concentration of cations per anions in the leach liquor that known as ionic strength are considered as significant parameters, which affect the pH factor [38].
Pulp density
It is an important parameter to establish the metal leaching process, it is also called solid to liquid ratio. The uranium leaching process by the fungal strain A. nidulans achieved an increase in the leachability by increasing the pulp densities in the fungal growth media up to 3% at room temperature and pH value equals 3, which recorded the highest percentage leaching efficiency of uranium as in Fig. 18. Moreover, the obtained data from the ore sample with the maximum bioleachability value of uranium exhibited at 3% of the studied sample, which approached 82% by A. nidulans. Above an optimum pulp density value over than 3%, the decrease in leaching efficiency of uranium was observed. The accumulation of heavy metals at high concentrations may be lead to the destruction of cell membrane integrity as well as the growth and metabolism of fungi is hindered [39]. This may be in view of an oxygen transfer deficiency, shortage nutrition supplied to the fungi, or cell distortion by increasing the ore concentrations, as demonstrated by Mohapatra et al. [40]. The increase in the leached metals concentration may inhibit both the growth of the microorganisms and the production of fungal acids and their metaboiltes used in the bioleaching process.
Incubation periods
The contact time between fungi and medium components as well as the supply of oxygen, making the fungi to reproduce uniformly and improve the efficiency of culture and leaching process. The leaching efficiency of uranium by A. nidulans has been significantly affected by the factor of incubation periods. The most preferable leachability happened upon pH 3, 3% pulp density, and the period of incubation as 7 days, whereas the solubilized amount of uranium from the sample was 80%. After the incubation period of 7 days the amount of solubilized uranium insignificantly decreased by increasing the incubation period up to 11 days Fig. 19. The obtained data agreed with El Sayed results [41], where he observed that were the optimum incubation periods for A. niger and A. fumigatus eight and six days respectively to solubilize uranium and rare earth elements from gibbsite shale sample. A sufficiently long period should be provided for microbial growth and metabolites production in order to achieve maximum leaching efficiency. Only when the leaching conditions match the fungi's optimal growing conditions can maximal uranium yields be reached.
Incubation temperature
Temperature affects bioleaching processes via two competing factors; (i) the enhancement in reaction rate usually brought about by an increase in temperature, and (ii) an increase and then decrease in microbial activity as the temperature increases and approaches the upper tolerance limit of the microorganisms. Consequently, the suitable temperature range for bioleaching process should be adopted in order to provide an optimal condition for microbial growth and reaction. The leaching of uranium from its ore was exhibited extremely affected by the factor of incubation temperature, which recorded the maximum leaching efficiency at 30 °C. At this temperature, A. nidulans solubilized 82% of the uranium found in the ore sample with previous optimum conditions; pH value equals 3, 3% pulp density, and 7 days as an incubation period Fig. 20. At this incubation temperature, the final pH value of the medium shifted toward acidity. El Sayed noticed that bioleaching of heavy metals occurred its maximum value at 30 °C for A. niger and 35 °C for A. fumigatus [41]. At superior temperatures higher than 30 °C (40 and 45 °C), the decrease of the fungal metabolic rate was observed and the fungus could even be unable to survive [40]. The previous optimum conditions were applied upon the uranium bioleaching process, that proved previously as; pH value equals 3, 3% pulp density, 7 days incubation time, and 30 °C incubation temperatures, upon one kg. of the studied sample using A. nidulans, the uranium recovery achieved 80% leaching efficiency as a high bioleaching efficiency. The obtained percentage is closes to the data obtained by Al-Sohaibani [42], who concluded that the Aspergillus species attained 75% of uranium recovery. Mishra et al. [43, 44] and Zhou et al. [45] using two Aspergillus species isolated from water samples contain uranium that collected from mines and evaluated that the maximum recovery of uranium. They demonstrated that Aspergillus flavus and Curvularia clavata, recorded 59% and 50%, respectively of uranium.
From the above study, the advantageous of bioleaching technique is summarized as it is considered as one of the most eco-friendly process, the microorganism A. nidulans is already isolated from the rock sample witch makes it more adapted than other and economically, i.e. leaching process carried out by metabolism of fungi which secrete appropriate organic acids (citric, oxalic, …ect.) in addition to amino acids, enzymes. However, this technique is nonselective for uranium because the metabolism of fungi able to dissolve other valuable metals. Although the conventional acid leaching achieve high uranium leaching efficiency 95.0%, and dramatically alkaline leaching efficiency reach to 68.5%, With the examined ore sample, the disadvantages of both acidic and alkaline leaching can be summarized as the following: Acid consumption in carbonate–bearing ore which increases chemical costs and could render the process unprofitable. The drawback of sulphuric acid leaching is the necessity to use corrosion-resistant equipment and pipelines which make process more costly. The presence of gangue minerals such as silica and alumina affect leachbility. Both of carbonate alkaline and sulphuric acid leaching is not favorable environmentally, because when they applied in situ leaching, sulphuric acid leaching leads to formation of several compounds in the groundwater and in carbonate leaching introduces substances (e.g., Ra and Se) to a solution capable of migrating over extended distances in weakly alkaline medium IAEA [46].
5. Conclusion
The comparison of uranium leaching sandstone ore sample of Abu Thor, Southwestern Sinai by using different leaching techniques has been studied. The conventional uranium leaching efficiency reached 95.0% with sulfuric acid leaching at a better condition of 200 g/l H2SO4 concentration for 180 min agitation leaching contact time with (3/1) liquid/solid ratio at 85 °C leaching temperature. This leaching efficiency decreased to 68.5% only using 150 g/l of (3:1) Na2CO3/ NaHCO3, 120 min leaching agitation contact time with 3/1 liquid/ solid ratio at 85 °C leaching temperature. The mechanism of uranium chemical leaching was determined by using kinetic models specially the shrinking core model, which was detected to be following the product layer diffusion process with acquiring apparent activation energy of 16.82 and 18.28 kJ/mol for sulpuric acid and carbonate leaching respectively. The nonconventional leaching technique of uranium by using the bioleaching process recorded 80% with the optimization pH value equals 3, 3% pulp density, 7 days incubation time, and 30 °C incubation temperatures. Consequently, the bioleaching process was recommended, which is, nonexpensive, economically and an eco-friendly process.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
NEA and IAEA, (2020) Uranium 2020: Resources, production and demand. OECD 2020 NEA No. 7551, nuclear energy agency organisation For economic co-operation and development
Haneklausa N, Bayokb A, Fedchenkoc V (2017) Phosphate rocks and nuclear proliferation. Sci Glob Secur 25(3):143–158
Ammar F, Omar S, El Sawey E (2016) Genetic affiliation of gold and uranium mineralization in el-missikat granite, Central Eastern Desert. Egypt Nucl Sci Sci J 5:33–47
Aziz A, Sief R, Ghieth B, Kaiser M (2020) Black sand deposits; their spatial distribution and hazards along the northern coast of Sinai Peninsula Egypt. J Appl Geophys 183:104219. https://doi.org/10.1016/j.jappgeo.2020.104219
Youltona BJ, Kinnaird JA (2013) Gangue–reagent interactions during acid leaching of uranium. Miner Eng 52:62. https://doi.org/10.1016/j.mineng.2013.03.030
Anand K (2020) Rao Bhaskar Paul, Sreenivas T (2020) Morphological variations during carbonate leaching of uranium from indian alkaline host rocks. Trans Indian Inst Met 73(8):2069–2080. https://doi.org/10.1007/s12666-020-01983-z
Abhilash B, Pandey D (2013) Microbially assisted leaching of uranium—a review. Miner Proc and Extrac Metal 34:81–113. https://doi.org/10.1080/08827508.2011.635731
Bakry AR, El-Hady SM (2015) Alkaline selective leaching of uranium from carbonate-rich black shale Wadi Naseib, southwestern Sinai. Egypt CTAIJ 10(6):266–275
Abu Khoziem HA (2017) Mineralogical characteristics and leachability of some valuable metals from Abu Zienema poly mineralized carbonaceous shale, southwestern Sinai. Egypt J Sedimentol 23:57–70
Oraby A, El- Skeikh E, Salah W, El- Saied F, El Gendy H, Ismaiel D (2019) Recovery of uranium and copper from mineralized Dolostone, Gabal Alluga, southwestern Sinai, Egypt. J Radiat Res Appl Sci. https://doi.org/10.1080/16878507.2019.1594095
Abdel Wahab S, Rezik A, Abu Khoziem H, Khalid E, Abdellah W (2019) Kinetics of uranium carbonate leaching process from carbonaceous shale, southwestern Sinai. Egypt Euro Mediterr J Environ Integr 4:19. https://doi.org/10.1007/s41207-019-0106-0
Kiegiel K, Miskiewicz A, Gajda D, Sommer S, Wolkowicz S, Zakrzewska-Koltuniewicz G (2018) Uranium in Poland: resources and recovery from low-grade ores. IntechOpen. https://doi.org/10.5772/intechopen.72754
Singh RL (2017) Applied environmental science and engineering for a sustainable future. Springer Science+Business Media, Singapore. https://doi.org/10.1007/978-981-10-1866-4
Amin MM, Elaassy IE, El-Feky MG, Sallam AM, Talaat MS, Kawady NA (2014) Effect of mineral constituents in the bioleaching of uranium from uraniferous sedimentary rock samples, Southwestern Sinai. Egypt J Envi Radio 134:76–82. https://doi.org/10.1016/j.jenvrad.2014.02.024
Castro L, Bl´azquez ML, Mu˜noz FGJA (2020) Bioleaching of phosphate minerals using earth elements. Metals (Basel). https://doi.org/10.3390/met10070978
Liang X, Gadd GM (2017) Metal and metalloid biorecovery using fungi. Microb Biotechnol 10:1199–1205. https://doi.org/10.1111/1751-7915.12767
Amin MM, Elaassy IE, El-Feky MG, Sallam AM, Talaat MS, Kawady NA (2018) Recovery of uranium from low-grade ore using microorganism isolated from uraniferous rock sample. Sep Sci Technol 53(14):2232–2237. https://doi.org/10.1080/01496395.2018.1437182
Abhilash Mehta K D, Kumar V, Pandey B D (2007) Bio-hydrometallurgical approach in processing of low grade Indian uranium ore in column reactor. Biohydrometallurgy. 531–536. https://www.mineng.com/biohydromet07/paps.html
Barron T (1907) The topography and geology of western Sinai Egypt. Surv Dept Cairo. 24:241. https://doi.org/10.1007/978-3-030-15265-9_6
Wiessbroad T (1969) The Paleozoic of Israel and adjacent countries (Part 2). The Paleozoic outcrops of southwestern Sinai and their correlation with those of southern Israel. Geol Surv Israel 48:32
Soliman MS, Abu El Fetouh MA (1969) Petrology of carboniferous and sandstone in West Central Sinai. Egypt J Geol UAR 13:43–61
Beyth M (1981) Paleozoic vertical movements in Um Bogma Area, southwestern Sinai: reprinted for private circulation from the American Association of petroleum Geologist. 65(1)
Hussein HA, Abdel-Monem AA, Mahdy MA, El-Assay IE, Dabbour GA (1992) On the genesis of surficial uranium occurrences in west Central Sinai. Egypt Ore Geol Rev 7:125–134
Said R (1971) Explanatory notes to accompany the geological map of Egypt. Geol Surv Egypt Cairo 56:123
Shapiro L, Brannock W W (1962) Rapid analysis of silicates, carbonates and phosphate rocks, US Geol Surv Bull 114 A
Mathew K J, Burger S, Ogt S V, Mason P M, Narayanan U I (2009) Uranium assay determination using Davies and Gray titration. Processing the eighth international conference on method and applications of radio analytical chemistry (Marc Viii) Kaailua-Kona, Hawaii, 5
Santos EA, Ladeira ACQ (2011) Recovery of uranium from mine waste by leaching with carbonate-based reagents. Environ Sci Technol 45:3591–3597. https://doi.org/10.1021/es2002056
Sreenivas T, Chakravartty JK (2016) Alkaline processing of uranium ores of Indian origin. Indian Inst Metals 69(1):3–14. https://doi.org/10.1007/s12666-015-0548-2
Desouky OA, Mira H, El-Feky MG, Abed NS (2018) Selective leaching of uranium from recent deposits of Wadi El Reddah stream sediments northeastern Desert Egypt. Material Science An Ind J 16(2):132
Orrego P, Hernández J, Reyes A (2019) Uranium and molybdenum recovery from copper leaching solutions using ion exchange. Hydrometallurgy 184:116–122. https://doi.org/10.1016/j.hydromet.2018.12.021
Zeng S, Li J, Tan K, Zhang S (2018) Fractal kinetic characteristics of hard-rock uranium leaching with sulfuric acid. R Soc open sci 5:180403. https://doi.org/10.1098/rsos.180403
Levenspiel O (1999) Chemical reaction engineering. Wiley, New York, p 664. https://doi.org/10.1021/ie990488g
Crundwell FK (2013) The dissolution and leaching of minerals: mechanisms mythy and misunderstandings. Hydrometallurgy 139:132–148. https://doi.org/10.1016/j.hydromet.2013.08.003
Dusengemungu L, Kasali G, Gwanama C, Mubemba B (2021) Overview of fungal bioleaching of metals. Environ Adv 5:100083. https://doi.org/10.1016/j.envadv.2021.100083
Srichandan H, Mohapatra RK, Parhi PK, Mishra S (2019) Bioleaching approach for extraction of metal values from secondary solid wastes: a critical review. Hydrometallurgy 189:105122. https://doi.org/10.1016/j.hydromet.2019.105122
Okoh MP, Olobayetan IW, Machunga-Mambula SS (2018) Bioleaching, a technology for metal extraction and remediation: mitigating health consequences for metal exposure. Int J Dev Sci 7(7):2103–2118
Kawady A N (2021) Biological solubilization and sorption of Uranium from sample at Abu Thor area, southwestern Sinai, Egypt using Aspergillus nidulans. Biomass Conversion and Biorefinery. In progress
Muddanna NM, Baral SS (2019) A comparative study of the extraction of metals from the spent fluid catalytic cracking catalyst using chemical leaching and bioleaching by Aspergillus niger. J Environ Chem Eng 7:103335. https://doi.org/10.1016/j.jece.2019.103335
Jang HC, Valix M (2017) Overcoming the bacteriostatic effects of heavy metals on Acidithiobacillus thiooxidans for direct bioleaching of saprolitic Ni laterite ores. Hydrometallurgy 168:21–25. https://doi.org/10.1016/j.hydromet.2016.08.016
Mohapatra S, Bohidar S, Pradhan N, Kar R N, Sukla L B (2007) Microbial extraction of nickel from Sukinda chromite overburden by Acidithiobacillus ferrooxidans and Aspergillus strains. Hydromet 85–93. http://ore.immt.res.in/handle/2018/1273
El Sayed H (2012) Fungal leaching of some heavy metals from soil sediments, South Western Sinai, Egypt (M.Sc. thesis). Faculty of science, botany department, Zagazig University
Al-Sohaibani SA (2011) Heavy metal tolerant filamentous fungi from municipal sewage for bioleaching. Asi J Biotech 3:226–236. https://doi.org/10.3923/ajbkr.2011.226.236
Mishra A, Pradhan N, Kar RN (2009) Microbial recovery of uranium using native fungal strains. Hydromet 95:175–177. https://doi.org/10.1016/j.hydromet.2008.04.005
Omara S, Conil R (1965) Lower carboniferous foraminifera from Southwestern Sinai, Egypt. Annals Soc Geo Belgique 88:221–240
Zhou Y, Huang X, Huang G (2008) Cu and Fe bioleaching in low grade chalcopyrite and bioleaching mechanisms using Penicillium janthinellum strain GXCR. Chin J Biotech 24:1993–2002
IAEA (2001) Manual of acid in situ leach uranium mining technology (2001): International atomic energy agency, IAEA-TECDOC-1239
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
R M A: Do the chemical leaching practical part. Write the part of the chemical leaching. N A K: Do the bioleaching practical part. Write the part of the bioleaching. A E A A: Collect the sample from the field. Write the geological part. O R S: Collect the sample from the field. Write the geological part.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Attia, R.M., Sallam, O.R., Abbas, A.E.A. et al. Comparative evaluation of chemical and bio techniques for uranium leaching from low grade sandstone rock sample, Abu Thor, southwestern Sinai, Egypt. J Radioanal Nucl Chem 331, 5675–5689 (2022). https://doi.org/10.1007/s10967-022-08621-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08621-6