Abstract
During decommissioning of nuclear facilities, possibly contaminated asbestos containing materials (ACM) emerge. In this work, we propose an analytical method to characterize ACM contaminated with alpha and beta nuclides by microscopic (light and electron microscopy) and radioanalytical techniques. For this purpose, a chromatographic separation is applied after decomposition of ACM by a lithium borate fusion at 1065 °C. The subsequent separation is performed with UTEVA-TRU-Sr chromatographic resins. Recovery rates for analyzed radionuclides were on an average of 80–90% for Am, Cm, Pu isotopes, and Sr-90. Compared to sample pre-treatment with hydrofluoric acid, the lithium borate fusion proves more suitable, while providing higher working safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asbestos containing materials (ACM) were commonly used as building and insulation material in the 20th century due to their mechanical and chemical properties, as well as their heat resistance. These are essential, e.g. for insulation of high temperature ovens and their exhaust gas lines. Smaller devices that are operated at elevated temperatures, such as industrial scale microwave oven systems, also occasionally contain ACM. Nowadays, due to their hazardous properties, asbestos and ACM are banned from modern construction sites. Nevertheless, since many important industrial and technological buildings were constructed in the seconds half of the last century, ACM can be found in rather large quantities [1,2,3,4,5,6,7].
ACM are uncovered during removal, deconstruction, and decommissioning procedures of closed down facilities and must be correctly disposed. This complicates the decommissioning of old nuclear facilities, as the asbestos can be contaminated with radioactive isotopes, which have to be addressed separately. Thereby, various isotopes might be expected, i.e. activation products, actinides and their fission products, as well as radionuclides from radiopharmaceutical research. Hence, it is of great importance to find an analytical way to determine the radioactivity of contaminated ACM, which fulfills safe working conditions and yet is practical in a typical radioanalytical laboratory.
Asbestos is toxic not because of its elemental composition, which is rather inconspicuous, e.g. Chrisotyle consists of Mg3(Si2O5)(OH)4 [8], but because of its structure. It forms nanoscale, long fibers which can float in air when released by drilling or cutting of the material. Breathing in those fibers can cause an aggressive form of lung cancer, namely asbestosis [9]. Therefore, a mere chemical analysis for confirmation of the presence of asbestos is not sufficient. The analytical method must provide additional information about the geometry of the fibers in question. This is usually done by scanning electron microscopy (SEM) in combination with elemental analysis by energy-dispersive x-ray (EDS) detection and light microscopy. In practice, most laboratories that perform asbestos analysis have the capacity neither to handle radioactive materials nor to perform radionuclide analysis, and vice versa.
The goal of any deactivation step is therefore primarily to destroy the fibrous composition of the material. Most chemical dissolution procedures for asbestos contain either hydrofluoric acid (HF), alkali or borate fusion processes, or leaching methods with acid combinations [8, 10]. While HF treatment is expected to provide complete dissolution of ACM, it is itself a highly toxic chemical. In most chemical laboratories, it is possible to implement necessary safety procedures for HF, but this complicates the analytical process. Combinations of less toxic acids, such as oxalic acid, hydrochloric and perchloric acid, nitric acid, as well as phosphoric acid have been investigated for their use in asbestos deactivation [11, 12]. Most of those methods were either time consuming or rather ineffective when it comes to complete dissolution of the ACM.
In this work, we propose a safe and fast method to process radioactivity-containing ACM in the laboratory.
Theory of asbestos processing
Given its high resistance against many chemicals, acid treatment results in leaching of secondary elements from the silicate matrix. This includes especially Mg, but also the leaching of Na, K, and Ca. Though, for example, leached and thereby magnesium depleted chrysotile (serpentine type) asbestos can be considered asbestos-free from a chemical point of view, the fiber structures are still intact. Furthermore, those acid leaching processes are not able to dissolve Fe containing asbestos effectively, i.e. amosite (amphibole type) asbestos. An alkaline or borate fusion process is capable of dissolving asbestos completely, even though solubility is only guaranteed during the fusion process itself. This type of dissolution method is well known, frequently used in geology to decompose minerals completely [13,14,15], and has already been introduced for radiochemical applications [16,17,18,19]. At 900–1100 °C, the fusion agent forms a liquid phase of low viscosity, which act as a solvent for the sample material. Both the temperature and the lithium borate as a chemically aggressive medium play a crucial role. During the process, the entire sample is dissolved, thereby resulting in a homogeneous distribution of all components, including radionuclide tracers as well as analytes. During the cooling process, the molten salt forms a glass. Alternatively, pouring the melt directly into an acidic medium, e.g. HNO3 at room temperature, enables dissolution of the molten glass and thereby the release of the sample constituents into aqueous solution. For ACM, this method appears to be advantageous, since even the silicate matrix is dissolved completely during the fusion. The silicates tend to precipitate as silica gel, thus making subsequent analytical separation of the radionuclides more difficult. Therefore, polyethylene glycol with an average molecular weight of 6000 (PEG-6000, obtained Sigma-Aldrich) is added to the chosen acid to enhance the flocculation behavior of the SiO2, which then can be easily filtrated before entering subsequent separation steps.
To investigate the method’s capability for radioanalysis, ACM from an experimental incinerator currently under decommissioning, located at the Paul Scherrer Institute (PSI, Switzerland), was studied. Evaluation of the analytical methods is based on the effectiveness of the dissolution or leaching process, as well as on the recovery rates of different radionuclides, and the reproducibility of the method itself. The effectiveness of the proposed analytical method is based on a dry-ashing step to deactivate major part of the asbestos, thus enabling a lithium borate fusion with subsequent radionuclide separation and high tracer recovery rates.
Experimental
Characterization of asbestos
Four asbestos samples were taken from the experimental incinerator, approx. 1.6 m in diameter and 6 m in height, from its refractory lining and from the successive filter exhaust lines. Of the 1.6 m diameter of the incinerator, 0.7 m consisted of a cavity, which served as exhaust for the oven (see Fig. 1 for a schematic illustration of the structure).
Schematic layout of the experimental incinerator at Paul Scherrer Institute (PSI), Switzerland. The inner white area displays a cavity representing the exhaust line for the combustion gases and fumes. Victor50 (bauxite: approx. 50% Al2O3, 40% SiO2/TiO2/Fe2O3/P2O3) and JM23 (mullite: approx. 37% Al2O3, 44.4% SiO2, 15.2% CaO/TiO2/Na2O + K2O) are porous fire clays, which were in direct contact to the fumes. FibroCel and FibroM belong to the asbestos containing materials (ACM) that were investigated in this work. A steel ring enclosed the refractory lining
The outer sheathing was made of steel with a thickness of 8 mm, and could be decontaminated and decommissioned readily. Different building materials were assembled in multiple rings around the furnace cavity. The first ring from the inside out consisted of two different fireclays, namely Victor50 and JM23, both with a thickness of 115 mm. The volume in between the fireclays and the steel ring was filled with insulation material and consisted mainly of SiO2 with variable mineral compositions. The four ACM samples have the name ‘FibroM’ and ‘FibroCel’, highlighting the fibrous character of the insulation material (see SI, Fig. 2). FibroM is the major ACM, which as liner of the furnace compartment, while FibroCel was inserted as an interlayer in between fireclay and asbestos insulation. Its chemical composition was assumed to be 59% SiO2, 30% MgO, 10% CaO/FeO for both silicate minerals based according to the manufacturers of those brands. FibroCel is representing a diatomite type of material (kieselgur) and FibroM the pure asbestos (chrysotile). However, microscopic and elemental analysis during this work proved ACMs to contain several types of asbestos, chrysotile and at least one amphibole asbestos, i.e. amosite.
For the first characterization of the fibers within the ACM, a polarized light microscopy (Zeiss Axio Imager, Axiocam208c; short: PLM) and scanning electron microscopy (Zeiss Sigma 300 VP; short: SEM) with an energy dispersive x-ray detector (Oxford Xmax 50; short: EDS) were used. These procedures were performed by an external partner laboratory specialized in the analytics of asbestos in building materials (Suva, analytical division). With 10–50 mg of each material, only small amounts were necessary for the microscopic analysis. Furthermore, only an aliquot of the weighed portion was used because of the high asbestos content of the materials. Chrysotile fibers are easily detectable by both microscopic techniques due to their characteristic form and shape. Fiber bundles can be observed with low magnification in the PLM, and the interconnection of the fibers can be resolved well. By using polarized light, reliable identification and discrimination from different fiber types was possible. Compared to usual glass fibers, asbestos fibers shift the wavelength of incident polarized light producing a visible change in color (polarization of light) [20, 21].
Compared to PLM, SEM images provide higher magnification and more information on the fiber structure. Due to the small wavelength of the electrons used, a magnification level down to sub-micrometer scale is achieved. While identification can be performed with PLM, effects of the chemical and mechanical treatment such as chrysotile fiber bundles torn apart or amosite needle ends split are easier to detect. In addition, SEM EDS provides insight into the elemental composition, either via scanning over a surface area or by point selection. Even though interferences might occur, the characteristic x-rays can be used for elemental analysis.
For analysis of ACM, only small amounts were necessary for identification with microscopic techniques. Only 10–50 mg were added into aqueous solution and filtered by means of a polycarbonate gold-coated membrane filter (0.2 micro, 40/20 nm coating, 25 mm). Asbestos fibers were kept onto the filter to be transferred into the SEM. For analysis with PLM, the filter was dried at 70 °C for 30–60 min and the residue was removed. Approximately 1 mg of the powder was mixed with the embedding medium (immersion oil with refraction index n = 1.55 for chrysotile) and placed onto the slide, on top of which a coverslip was placed. To investigate the other asbestos types, another slide with triacetin (refraction index n = 1.43) had to be prepared, since all asbestos types showed an easy to detect optical contrast in this medium.
Chemical processing and asbestos dissolution
Processing of asbestos samples in the laboratory was challenging, both because of the resistance of the fibers against most chemicals and because of relevant safety precautions. In most cases, total digestion with hydrofluoric acid (HF) is applied to determine the elemental composition of pure asbestos. Commonly used in geology to characterize minerals accurately, the addition of HF provides a way of dissolving silica. Corresponding reaction of fluorine with silicon dioxide leads to the formation of silicon tetrafluoride, which evaporated as volatile gas [22]. When taken up in acid, a precise elemental characterization can be carried out subsequently, apart from the silicon content. The disadvantage of such a digestion method is the use of hydrofluoric acid as a highly toxic chemical. In addition, complete dissolution of ACM that contains impurities from various building materials is probably not guaranteed. Generally, when working with ACM, necessary safety precautions include sample handling in a fume hood as well as wearing a respiratory mask (FFP3).
For the dissolution steps proposed in this work, all chemicals used were at least analytical grade. Fusion using lithium borate offers a good alternative to HF when combined with the PEG addition, yet requires safety measures regarding work with a high temperature oven. The detailed (pre-) treatment and chemical separation is displayed in Fig. 2.
Preparation scheme for asbestos containing materials (ACM), including chemical separation with UTEVA-TRU-Sr resins and deposition procedure after fusion in lithium borate molten salt (deposition technique from [23])
Prior to the fusion, a dry-ashing step at 900 °C for 6 h was performed to destroy organics within the sample, which could cause superheating and spilling due to CO2 production. Chrysotile asbestos was decomposed to short-chained SiO2 during this process, while amphibole types remained untouched.
Recovery tracers (from Eckert&Ziegler) were added to all samples before digestion. The tracers included Pu-242, Am-243, and Sr-85 for the Pu and Am fractions, and Sr-90, respectively. A mixture of lithium metaborate and lithium tetraborate [2–3 g LiBO2/ Li2B4O7 (80:20 w/w%), Spectroflux 100B from Alfa Aesar] was used to produce a molten salt at 900–1000 °C, in which various common building materials can be dissolved. To ensure this, the crucible has to be shaken to achieve complete mixing. The movement of the crucible is a critical step from a safety point of view and can be circumvented by using an automated digestion unit. The digestion procedure used in this work was performed using the Claisse© LeNeo© Fusion Instrument from Malvern Panalytial [24]. After performing the digestion, the still hot molten salt was poured into 4.5 M HNO3, resulting in rapid cooling, which led to the formation of a glassy material. The solution obtained, including the melt, was stirred at 190–200 °C for 1 h, to ensure complete dissolution. Slow cooling of the solution caused incomplete precipitation of the silicates. The added polyethylene glycol (PEG-6000, Sigma Aldrich) enhanced formation of long SiO2 chains, by which the filtration process is simplified. The flocculated SiO2 had to be removed from solution via filtration, as it inhibits further chemical separation due to clogging of the resins cartridges. This effect occurs for asbestos, since they consist mostly of silicate. To remove the corresponding silicate, the sample is filtered (Whatman™ GF/B on a Büchner funnel) and the filtrate is evaporated. This step is followed by uptake in 3 M HNO3 by heating to 150 °C, achieving the necessary acid strength for the subsequent column chromatographic separations. During the addition of the HNO3, silicate may precipitate once more, which must be filtered off to prevent clogging, as already mentioned above.
For comparison, both the fusion technique and a standard HF digestion procedure were performed with the same ACM samples. Therefore, ACM was placed into polytetrafluoroethylene (PTFE) beaker, and HF (25%) and HNO3 (65%) were added multiple times and heated to 150° C until dry. This procedure was repeated three times with concentrated HNO3, the following three additions consisted of concentrated HF alone. Since HF was added in excess and the subsequent steps require an HF-free environment, H3BO3 was added. With this step, a complete consumption of the HF can be ensured. The residue was taken up in 3 M HNO3 / 1 M Al(NO3)3.
Radiochemical separation
The separation procedure (see Fig. 2) was performed for both samples prepared by fusion and HF digestion. After filtration and uptake in 3 M HNO3 / 1 M Al(NO3)3, oxidation state of Pu was adjusted to + III by addition of 2 ml 1 M iron(II)dilsulfamate ((NH2SO3)2Fe) and 200 mg ascorbic acid. The color of the sample might change from clear/light yellow to blurry yellow/green. Triskem® cartridges UTEVA-TRU-Sr were stacked accordingly, attached to a vacuum box, and conditioned with 10 ml 3 M HNO3. The resins were loaded with the sample solution. The sample beakers were washed at least once with additional 5 ml 3 M HNO3 and also added to the column. After washing, the cartridges were separated. While U(VI) and Th(IV) isotopes were bound to the UTEVA resin, trivalent isotopes – Am, Cm, and Pu(III) – were attached to the TRU resin. Am and Cm were not retained on the UTEVA at any HNO3 condition. Unimpeded by UTEVA and TRU, Sr(II) bound to the Sr resin.
Both elements, U and Th, bound well to the UTEVA resin at 3 M HNO3. Yet another washing step with 10 ml 8 M HNO3 helped to remove interfering elements and led to stronger bonding of U and Th to the substrate. Selective elution of Th was achieved by addition of 10 ml 4 M HCl, where U was still retained. According to Triskem/Eichrom, stripping U off UTEVA works well with diluted HNO3 (0.01 to 0.05 M). However, the use of 1 M HCl was stated to be more efficient, which we could confirm.
After loading Am, Cm, and Pu isotopes to the TRU resin, further washing was considered, if high amounts of Fe(III) were expected to be in the sample solution. This was indicated by a strong yellow stain of the resin. For an even better visual indication, a few drops of ammonium thiocyanate (NH4SCN) were added. If sufficient levels of Fe(III) were present, NH4SCN reacted with Fe to produce Fe(SCN)3, an intensely red complex. Washing was done by addition of 3 M HNO3, in which 100 mg of ascorbic acid has been dissolved. This step was repeated multiple times until the resin is decolorized. Removal of Fe was necessary for the upcoming deposition of the actinides for alpha spectrometry, since high loads of Fe cover the planchet with a black coating and reduced the signal or made detection impossible.
To oxidize Pu(III) to Pu(IV), 104 mg NaNO2 was dissolved in 5 ml 3 M HNO3 and added to the TRU resin. The oxidation step was followed by another washing step with 5 ml 3 M HNO3 and 5 ml 0.5 M HNO3 to reduce the nitrate concentration for the upcoming HCl addition. To strip Am and Cm from the TRU resin first 3 ml 9 M HCl and 17 ml 4 M HCl was used. The last step for the TRU involved elution of Pu by adding of 20 ml 0.1 M ammonium hydrogen oxalate [C2H5NO4] solution. This complexing agent readily removed metal ions from the resin and thereby served as stripping step.
All eluted actinides were further prepared for alpha spectrometry. Calcination was performed by adding HClO4, H2SO4, and NaHSO4 to the solution and heating it to approx. 500 °C on a heating plate. For alpha spectrometry, uptake in a buffer solution is mandatory for follow-up deposition. Except for Th, the other actinides could be deposited by 60 min electrodeposition at approx. 1.2 V (10–12 V nominal voltage set on the power supply, which drops to 5–7 V under load) in a buffer system containing 4.2 ml H2O, 0.6 ml 1 M NaHSO4, and 5.2 ml 1 M Na2SO4. For Th, a more efficient deposition was achieved by slightly changing the buffer system to 1.6 ml 1 M NaHSO4 and 4.2 ml 1 M Na2SO4 [23]. After 60 min the deposition was stopped with 1 ml concentrated NH4OH. The current was maintained for an additional 1 min to ensure complete deposition. A short washing step of the planchets with 0.1 M NH4OH removed residual salts from the buffer system. Afterwards the planchets were dried at 250° C on heating plate or under an infrared lamp. Measurement of Pu-241 is particularly difficult, since measurement via alpha spectrometry is not feasible. Therefore, after measurement, the Pu containing planchets were placed into 8 M HNO3 at 90 °C for 30 min [25]. This process resulted in the release of Pu in solution and after another evaporation step, the Pu fraction was taken up in 2 ml deionized water, mixed with 18 ml of Ultima Gold AB, and measured by alpha-beta discriminating LSC (Hidex). A second measurement of the treated planchets with alpha spectrometry ensured complete recovery of the Pu.
Eluted Sr-90 was evaporated and taken up in 2 ml 25% p-Toluenesulfonic acid (IUPAC: 4-Methylbenzene-1-sulfonic acid). The solution was transferred to a scintillation vial and mixed with 18 ml of scintillation cocktail (Ultima Gold LLT).
Measurement techniques
For electro-deposited actinides, measurement times ranged from 50 to 70 h (Alpha AnalystTM, Mirion Technologies). For Sr-90 determination, a three-window method was applied to measure the tracer nuclide Sr-85 (A-window), and to distinguish Sr-90 (B-window) from the ingrowing daughter Y-90 (C-window). Measurements were started less than 3 h after separation of Y-90, evaporation, and uptake in LSC cocktail. That way, the ingrowth of the interfering daughter nuclide was initially as low as possible and a clean beta spectrum was obtained (Packard Tri-Carb®). The first measurements were normally performed during the first 3 days after separation to observe the continuous ingrowth of Y-90. After waiting for 3 weeks, the samples could be measured to obtain once again the complete beta spectrum of Sr-90 and Y-90.
Results and discussion
Microscopic analysis
Figures 3 and 4 show examples of PLM and SEM examinations. Microscope images and element-specific element dispersive x-ray (EDS) spectra were taken from ACM of the waste incinerator.
The morphology of the fibers clearly indicate that they are asbestos. The fiber bundles typical for serpentine asbestos, i.e. chrysotile, are clearly visible in both PLM and SEM measuring techniques (see Fig. 3 and 4). It is notable that the detection of the fibers was possible with low effort, although only a small amount, between 10 and 50 mg, of the material was used for the examinations. This is an indication that a larger quantity of the material has a correspondingly high density of asbestos fibers per mass. In addition to the characteristic chrysotile fibers, different types of asbestos were found in the investigated samples. These include, on the one hand, rod-shaped fibers, which are also present in bundles, and on the other hand very long needles, which can have a length of > 100 μm. These needles represent amphibole type asbestos, which could be specifically identified as amosite by analysis of the elemental components with EDS (see Fig. 5). No significant difference can be seen between the acid-processed and original samples in terms of morphology. Both the fiber bundles and the needles show no obvious change, except for the fact that some of the fibers seem to have broken end pieces. The reason for this is most probably the mechanical stress during processing (continuous stirring by means of a stirring bar in the beaker).
Since a final determination of the material as ACM cannot be based on morphology alone, other properties of the asbestos must be identified using different analytical techniques. This step is necessary because there are many other types of fibers besides asbestos fibers that may have a similar appearance. Sheet silicates (phyllosilicates) as well as chain silicates can be reliably distinguished from other fiber types based on the polarization of the light in the PLM. A characteristic property of these layered silicates is to influence polarized light by rotating the polarization lane (direction of wave oscillation). When linearly polarized light is directed onto such a polarizer, a contrast image is obtained in a visible light range. An example of this is asbestos, which is a polarizer through the layering of silicon oxide and other elementary components (e.g. magnesium or iron oxide) in a crystal lattice. This shift is creating a blue colored fiber bundle on purple background (see Fig. 4). The effect does not only occur for chrysotile, but also for amosite asbestos. The characteristic needle shape of amosite is easily distinguishable from the chrysotile fiber bundles.
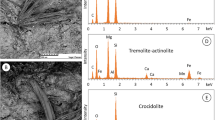
The EDS spectra provide additional information on the elemental composition of the asbestos fibers. Subsequent Fig. 5 shows EDS spectra for chrysotile and amosite.
The characteristic Kα lines, which were used for elemental analysis, are located at 1.25 keV, 1.74 keV, and 6.39 keV for Mg, Si, and Fe, respectively. The Au signal (Mβ line at 2.20 keV) derives from the gold filter used during the sample preparation and is not part of the sample. The Si and the O Kα signals can be seen, indicating the occurrence of Si-O bonds, and thereby the presence of silica based material. Furthermore, the Mg Kα line can be found for both chrysotile and amosite. Besides morphological evidence on fiber and needle asbestos types, the elemental composition can be used as secondary tool for positive identification. The high Mg content within the phyllosilicate chrysotile is a characteristic trait, which can be used for identification. In addition, EDS of the needle shaped fibers demonstrates the occurrence of Fe Kα signals, providing additional evidence of the presence of amosite (see Fig. 5).
Radiochemical analysis
Figures 6, 7 and 8 show the measurement results for both sample preparations (lithium borate fusion and HF). Each figure compares the activity concentrations of all analyzed radionuclides obtained by the two different digestion methods, fusion and HF digestion, for one ACM sample. Detailed, tabulated results and chemical yields are given in the Supplementary Information (SI), Table 1. All samples were analyzed at least twice to increase the statistical confidence. For Sr-90 and the actinides, separation and analysis works out well for utilization of three chromatographic columns in series.
Sr-90 recovery rates range from 61 to 92% with an average of (86 ± 7) % (N = 20). Comparable to Sr-90, both Am as well as Pu isotopes are separated with high selectivity. For the all analyzed asbestos samples, recoveries for the Pu fraction averaged (97 ± 4.5) % (N = 15), with the lowest recovery of 83% for the low-level asbestos FibroM-08. A higher variability and a lower recovery rate were observed for the Am/Cm fraction. On average, (81 ± 9) % was retrieved (N = 14). The determined recoveries were sufficient for quantitative evaluation of the radionuclides.
The high recoveries demonstrate the reproducibility of the separation method. For Sr-90, neither the preceding UTEVA nor the TRU resin is problematic in regard of the chemical yield, which is to be expected for the alkaline earth metals in their oxidation state of +2. Introduction of Sr dissolved in 3 M HNO3 appears unproblematic. Residual Y-90 is eluted from the resin with the addition of 8 M HNO3. This way, relatively high recovery rates are achieved for this multi-nuclide separation scheme with stacked columns. For the Pu fraction, high recoveries confirm the uncomplicated separation on the TRU resin after adjustment of Pu oxidation state to + 3. Complete reduction of Pu(IV) can be assumed, as plutonium in higher oxidation states would readily bind to the preceding UTEVA resin. The slightly lower yields for the Am/Cm fraction can be explained by slight losses on the UTEVA resin. In this case, the concentration of the 3 M HNO3 is slightly out of the optimal range, as the highest sorption of Am(III) on the UTEVA resin is demonstrated at 1–2 M HNO3 [26].
Distribution of radionuclides in insulating materials
Regarding the radioactive content of these ACM samples, a clear trend for the different insulating materials can be identified. Each figure compares the activities analyzed radionuclides depending on the digestion method used. The highest activity concentrations are found in the FibroM-05 with (70 ± 7) Bq/g (see Fig. 6), the lowest in FibroM-08 with (2.8 ± 0.9) Bq/g (see Fig. 7). In terms of overall activity in the middle, but with the greatest uncertainty, is FibroCel with (44 ± 15) Bq/g (see Fig. 8). These numbers reflect the activities found in the samples prepared by fusion digestion. Compared to these numbers, the samples digested with HF show in average only (56 ± 1.8) % of the total activity found with the other method. Therefore, when looking at the total activity, it becomes apparent that only just over half the sample could be dissolved and extracted.
When averaging the results of the fused samples, FibroM-05 shows elevated Sr-90 activity concentrations of (26.7 ± 0.8) Bq/g, while FibroCel and FibroM-08 both contain lower amounts with (25 ± 14) Bq/g and (0.7 ± 0.1) Bq/g, respectively. The reduction in detectable Sr-90 after HF digestion is greatest for FibroCel with (5.3 ± 0.3) Bq/g (2% of the activity determined by fusion).
This accounts for both the Am/Cm and the Pu fraction. Found activity concentrations for Pu-238 range from (0.02 ± 0.02) Bq/g to (1.5 ± 0.2) Bq/g and for Pu-239 + Pu-240 from (0.33 ± 0.05) Bq/g to (5.4 ± 0.9) Bq/g. In addition to the alpha emitting Pu isotopes, Pu-241 shows even higher activity concentrations of (1.3 ± 0.4) Bq/g to (21.5 ± 2.6) Bq/g. Even though the identified trend for the different materials is proving true for all analyzed nuclides, the elevated activity concentrations of the Cm isotopes for FibroM-05 is noteworthy. The asbestos sample shows elevated (0.007 ± 0.001) Bq/g and (0.74 ± 0.33) Bq/g for Cm-242 and Cm-243 + Cm-244, respectively. Likewise, lower activity concentrations are found for samples digested via HF, with a consistent reduction to (32–55) % for the Am/Cm fraction. In general, no trend can be identified for the various radionuclides, since the reduction for all analyzed isotopes ranges within the uncertainties between 25% and 71%. Therefore, this observation suggests an influence of the digestion method rather than a nuclide-specific property.
These results indicate a high permeability of the asbestos filled segments within the thermal insulation of the incinerator. Apparently, both Sr-90 and actinides were able to migrate further into the material, which was in contact with the smoke and exhaust from the oven. Furthermore, the present results suggests an inferior dissolution and extraction of radionuclides by HF compared to the fusion process. This is further discussed in the following section.
Distribution in the sample treatment
Results of the HF treated asbestos show a distinct difference when compared to samples prepared by fusion. In comparing the two methods, the yield for the HF digestion is consistently lower than the proposed lithium borate method. While the tracer-based recoveries appear to be similar for all analyzed radionuclides and samples (90%, 87%, 94% for the Pu fraction; 72%, 85%, 12% for the Am fraction; 90%, 85%, 84% for Sr90), most activity concentrations for the HF differ from the ones observed for the fusion. In particular, for the highly active asbestos sample FibroM-05, activity concentrations are shown to be 36 to 71% lower for all radionuclides analyzed (see Fig. 6). The low recovery of FibroCel can be explained by problems with the electrodeposition of the Am. For FibroM-08 and FibroCel, 2 of 6 and 3 of 6 isotopes show elevated activities, yet without comprehensible correlation between the two samples (see Figs. 7 and 8). The average for all representative values is (46 ± 12) %.
Even though HF treatment is considered an effective treatment of silicate containing samples, the lower activity concentrations indicate incomplete digestion. Apart from the chemical treatment, the properties of the analyzed ACM might explain the worse dissolution behavior in HF. Both the FibroM series and the FibroCel are aged samples, consisting of a mixture of building materials. When the sample arrived in the laboratory, they were already processed. During the decommissioning of the incinerator’s exhaust lines, the material might have not been separated completely from the other building materials, i.e. fireclays. Cross-contamination of different material types might have led to a mixture of silicate based ACM with aluminum, magnesium, and iron rich casting concretes. The latter ones prove high chemical resistance towards HF treatment, thereby reducing the found activity concentrations. An explanation for the different behavior of the ACM during the chemical digestion might be ‘aging’ of the material, as the ACM is very likely to have undergone chemical changes during its 30 years of operation. As this material was installed as insulation material in the early 1970s left in place during the whole operation period. Variability in temperatures between incineration periods and downtime can also be considered critical for alteration of the building materials. Long-term heating combined with cooling periods might have led to refractory particles, in which radionuclides are incorporated. Changes in chemical structure over time are very likely to have occurred. Therefore, a purely chemical HF digestion might not able to completely dissolve the radionuclides fused with the fireclay. Unfortunately, as no spare building material from the construction had been retained, this hypothesis could not be further investigated.
By formation of a molten salt, the fusion is utilizing both high temperature and chemical dissolution, whereas practical use of HF is limited to maximum of 200° C when using PTFE equipment (melting point: 327° C). Even more, refractory particles might even form heat and chemical resistant layers encapsulating the asbestos within and thereby providing a shielding against mere chemical treatment. While HF proves highly efficient for dissolution of silicates, digestion of merged composites of insulation materials appears to be incomplete. The same applies to treatment with HF in combination with concentrated HNO3. Hence, for the ACM analyzed in this work, the lithium borate fusion is the more suitable method for complete sample dissolution and preparation.
Conclusion
Decommissioning of nuclear facilities provides manifold challenges, especially for the radioanalytical investigation and characterization of all the different types of building materials. For the insulation material used in an experimental incinerator for nuclear waste at PSI in Switzerland, the asbestos contains high levels of actinides and fission products. It appears that the fibrosis character of the asbestos allows a possibility of radionuclide incorporation. Thereby, the analysis of this material can provide insight into the history and helpful evidence on the radionuclide inventory of decommissioned nuclear facilities, as well as indication for necessary waste treatment measures.
Chemical preparation for radioanalysis is mandatory for analysis of ACM as soon as nuclide specific measurements of alpha or beta emitters have to be performed. By utilizing a lithium borate fusion process, we provide an effective method to decompose asbestos fibers in their mixture with filling materials. The samples are dissolved at 1065° C in the fusion process and the molten salt is poured into nitric acid, containing PEG-6000, to allow removal of SiO2 gel and subsequent radiochemical separation. The combination of UTEVA-TRU-Sr resins provides an ideal combination to separate Am, Pu, U, and Th isotopes, as well as Sr-90 in another trapping step only. Additionally, the method might be further extended to separate and measure additional radionuclides of interest, i.e. Fe-55 and Ni-63. While thermal pre-treatment needs additional time, fusion, filtration, and separation of samples can be done within 2 to 3 days of working time. Since use of HF can be avoided and the fusion process automated, the procedure ensures high working safety. Hence, this method is suitable for reasonably fast radioanalytical characterization of asbestos containing materials.
References
Albin M, Magnani C, Krstev S, Rapiti E, Shefer I (1999) Asbestos and cancer: An overview of current trends in Europe. Environ Health Persp 107:289–298. https://doi.org/10.2307/3434419
Chen TH, Sun XM, Wu LC (2019) High Time for Complete Ban on Asbestos Use in Developing Countries. Jama Oncol 5:779–780. https://doi.org/10.1001/jamaoncol.2019.0446
Cugell DW, Kamp DW (2004) Asbestos and the pleura - A review. Chest 125:1103–1117. https://doi.org/10.1378/chest.125.3.1103
LaDou J (2004) The asbestos cancer epidemic. Environ Health Persp 112:285–290. https://doi.org/10.1289/ehp.6704
Ogunseitan AO (2015) The asbestos paradox: global gaps in the translational science of disease prevention. 93:359–360. https://doi.org/10.2471/BLT.14.142307
Ross M, Virta RL(2001) Occurrence, production and uses of asbestos.Can Mineral:79–88
Virta RL (2002) Asbestos: Geology, Mineralogy, Mining, and Uses (trans: Interior USDot). U.S. Geological Survey. https://doi.org/10.3133/ofr02149
Sugama T, Sabatini R, Petrakis L (1998) Decomposition of chrysotile asbestos by fluorosulfonic acid. Ind Eng Chem Res 37:79–88. https://doi.org/10.1021/ie9702744
Mossman BT, Churg A (1998) Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Resp Crit Care 157:1666–1680. https://doi.org/10.1164/ajrccm.157.5.9707141
Pawelczyk A, Bozek F, Grabas K, Checmanowski J (2017) Chemical elimination of the harmful properties of asbestos from military facilities. Waste Manage 61:377–385. https://doi.org/10.1016/j.wasman.2017.11.041
Rozalen M, Huertas FJ (2013) Comparative effect of chrysotile leaching in nitric, sulfuric and oxalic acids at room temperature. Chem Geol 352:134–142. https://doi.org/10.1016/j.chemgeo.2013.06.004
Valouma A, Verganelaki A, Maravelaki-Kalaitzaki P, Gidarakos E (2016) Chrysotile asbestos detoxification with a combined treatment of oxalic acid and silicates producing amorphous silica and biomaterial. J Hazard Mater 305:164–170. https://doi.org/10.1016/j.jhazmat.2015.11.036
Cremer M, Schlocker J (1976) Lithium Borate Decomposition of Rocks, Minerals, and Ores. Am Mineral 61:318–321
Ingamells CO (1970) Lithium Metaborate Flux in Silicate Analysis. Anal Chim Acta 52:323–. https://doi.org/10.1016/S0003-2670(01)80963-6
Feldman C (1983) Behavior of Trace Refractory Minerals in the Lithium Metaborate Fusion-Acid Dissolution Procedure. Anal Chem 55:2451–2453. https://doi.org/10.1021/ac00264a064
Jäggi M, Eikenberg J (2009) Separation of 90Sr from radioactive waste matrices–microwave versus fusion decomposition. Appl Radiat Isot 67:765–769. https://doi.org/10.1016/j.apradiso.2009.01.032
Jäggi MR, Eikenberg M, J (2019) Fusion and chemical separation of Am, Pu and Sr from barite-concrete. J Radioanal Nucl Chem 322:1279–1285. https://doi.org/10.1007/s10967-019-06854-6
Trdin M, Necemer M, Benedik L (2017) Fast Decomposition Procedure of Solid Samples by Lithium Borates Fusion Employing Salicylic Acid. Anal Chem 89:3169–3176. https://doi.org/10.1021/acs.analchem.6b04980
Croudace I, Warwick P, Taylor RN, Dee S (1998) Rapid procedure for plutonium and uranium determination in soils using a borate fusion followed by ion-exchange and extraction chromatography. Anal Chim Acta 371:217–225. https://doi.org/10.1016/S0003-2670(98)00353-5
Hawkins JW, Hayes DC, Istone WK, Schmidt AF (1987) Asbestos Abatement.1. Sampling and Identification. Tappi J 70:233–235
Santee K, Lott PF (2003) Asbestos analysis: A review. Appl Spectrosc Rev 38:355–394. https://doi.org/10.1081/Asr-120024393
Spasiano D, Pirozzi F (2017) Treatments of asbestos containing wastes. J Environ Manage 204:82–91. https://doi.org/10.1016/j.jenvman.2017.08.038
Bajo S, Eikenberg J (1999) Electrodeposition of actinides for alpha-spectrometry. J Radioanal Nucl Ch 242:745–751. https://doi.org/10.1007/Bf02347389
MalvernPanalytical (2021) Claisse LeNeo Fusion Instrument. https://www.malvernpanalytical.com/en/products/product-range/claisse-range/leneo
Jäggi M, Rollin S, Alvarado JAC, Eikenberg J (2012) Determination of Pu-241 in nuclear waste slurries: A comparative study using LSC and ICP-MS. Appl Radiat Isotopes 70:360–364. https://doi.org/10.1016/j.apradiso.2011.10.005
Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Essling AM, Graczyk D (1992) Separation and Preconcentration of Uranium from Acidic Media by Extraction Chromatography. Anal Chim Acta 266:25–37. https://doi.org/10.1016/0003-2670(92)85276-C
Acknowledgements
We would like to acknowledge the experts from Suva, Lucerne, regarding asbestos analysis, for their help with identification and characterization of the asbestos-containing material. The authors thank the support from the Swiss Nuclear Safety Inspectorate (ENSI), contract number: CTR00836.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Köhler, F., Heule, M., Jäggi, M. et al. Microscopic and radioanalytical investigation of asbestos-containing decommissioning waste. J Radioanal Nucl Chem 331, 5411–5421 (2022). https://doi.org/10.1007/s10967-022-08544-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08544-2