Abstract
In the framework of the project RemoteALPHA, an optical scanning system for remote sensing of alpha emitters using radioluminescence is being developed. After the feasibility of the technique was proven, current work aims at improving the sensitivity for detection of low surface activities. As calibration standard, pitchblende minerals were prepared. Their surface count rate of 80 Bq cm-2 to 105 Bq cm-2 was measured by alpha-track-detection and alpha-spectroscopy. Subsequently, radioluminescence measurements were performed in a sealed chamber filled with different gas atmospheres. The radioluminescence signal was measured in UVC and UVA spectral ranges for all samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
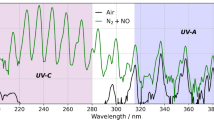
Since the usage of radioactivity in the civil or military sector began, radiation detection and protection have become very important [1]. Wide-area radiation monitoring is of high importance to public health and safety following the release of radioactive material from a nuclear facility due to an accident or even an attack. If contamination of the environment occurs, a fast and safe detection method is needed to perform protective measures based on knowledge of the contamination level and the kind of radiation. While gamma emitting nuclides are routinely screened by remote sensing techniques using helicopters or drones, contaminations by purely alpha-emitting radionuclides are harder to detect due to the short range of only a few centimetres of alpha particles in air [2, 3]. This requires ground based measurement, which is a time-consuming procedure and entrails a considerable risk of exposure of the emergency team not only to ionizing radiation but also to potentially hazardous materials, fire and debris [3]. Instead of detecting the alpha particles directly, detection of the radioluminescence of alpha particles in air is proposed to avoid these problems [2, 4]. Radioluminescence is the phenomenon that appears when a highly energetic ion e.g., an alpha particle, interacts with electrons of molecules of the surrounding medium (in air this is mostly nitrogen and oxygen). The energy transfer leads dominantly to ionization and excitation of the molecules. Secondary electrons above a few 10 eV, which are produced can collide with further gas molecules and lead to further ionization and more molecules that are excited. The excited molecules relax by emitting photons [5, 6]. The wavelengths of these photons correspond to the characteristic electron transitions. The majority of detectable photons originate from nitrogen transitions [5, 7]. Nitrogen has several emission lines in the range between 100 and 1000 nm. The strongest emission is in the UVA region of the electromagnetic spectrum with a wavelength of 337 nm [7]. For this reason, the 337 nm line is widely used in the remote detection of alpha particles [7, 8]. Another less intense wavelength is 254 nm located in UVC and the so-called solar blind region, where no UV-background from the sun disturbs the measurement [2, 9, 10].
The radioluminescence light of alpha particles generates approximately 19 ± 3 photons between 300 and 400 nm per MeV energy released in air at normal pressure (1013 hPa, 22 °C, relative humidity of 43%) [6]. The characteristics of the gas atmosphere should be well known due to its significant influence on the radioluminescence signal. This is mainly due to quenching effects of water vapour and oxygen [6, 11]. Sand et al. measured a six times higher amount of photons in pure nitrogen compared to ambient air [6]. The signal increase of the 254 nm wavelength is even higher if a small amount of nitric oxide (NO) is added to the pure nitrogen atmosphere [10, 12]. Kerst et al. [10] showed that the intensity of the signal is maximized for 50 ppm NO diluted in pure nitrogen. The light yield is about 25 times higher than the 337 nm line of nitrogen. In very pure nitrogen, a low ppm level of NO provides a significant increase in the UVC light yield. The limit of detection in UVC with 1 ppm NO is around 20 times lower than the limit of detection in pure nitrogen [2]. The use of small amounts of NO enables measurements of low activity samples. Krasniqi et al. [12] were able to measure an extended uranium source with an activity of 330 Bq by using an NO - amount of 3 ppm. Nitric oxide is an oxidizing gas that forms acids in the respiratory system. If human exposure is unavoidable, the exposure limit of 2 ppm over an 8-hour time weighted average, recommended by the Scientific Committee on Occupational Exposure Limits for Nitrogen Monoxide, should not be exceeded [13]. Therefore, it will be a challenge to use this approach in the environment.
For the detection of the radioluminescence signal, research groups used different optical systems. Some of them were listed by Crompton et al. [14]. Measurements were implemented under various conditions, with different nuclides and activities and diverse optical components. Baschenko [15] used a 37 MBq 239Pu source and measured the emitted luminescence by a monochromator and PMT whereas Giakos [16] used the same radionuclide with doubled activity but used an ICCD camera for detecting and mapping the radioluminescence photons.
Up to now, no radioluminescence measurements were performed on environmental samples such as pitchblende. Pitchblende is a naturally occurring radioactive mineral that contains mainly uranium dioxide and its radioactive daughters in equilibrium. Most of the daughters are alpha-emitters, which makes it a suitable material for our experiments. The utilization of environmental samples for radioluminescence measurements in the lab is a first approach to reach the goal of measuring contaminations on environmental surfaces. Due to the low specific alpha activity of pitchblende, it is also applied to explore the detection limits of the optical system used.
Experimental
Preparation
Pitchblende bearing ores from different locations with a comparatively high uranium content were cut into 5 mm thick slices with a micro-waterjet. The resulting surface is flat but not polished. The shape of the samples is irregular and their surface sizes range from about one to 15 cm2. The samples are listed in Sup. Table 1. A stone from Puy de Dôme in France was cut into the samples L, M, A and B. Sample S is from Uranium City in Canada. The remaining samples E, F, G, J and K are fractions of one single piece of ore originating from Wölsendorf in Germany. Sample J is the only polished sample. The small samples A, B, E, F, G and S are grouped to one sample in the following referred to sample Mix.
Alpha-track
For alpha-track-detection plastic detectors of the CR-39 type from TASL were used. After an exposure time of one to five minutes with alpha radiation from pitchblende samples, they were etched in 6 mol l-1 sodium hydroxide at 80 °C for three hours. The counting of tracks was performed by a microscope (Nikon LV-DAF) and the software ImageJ.
Grid ionization chamber
The grid ionization chamber from MABsolutions was filled with P10, a gas mixture of 90% argon and 10% methane, at a pressure of 1.025 bar. The accelerator voltage was set to 1600 V. Between the different measurements the chamber was flushed with air to remove radon from the previous sample. Measurement duration varied between 3000 and 14,000 s.
Optical system
The optical system consisting of lens, filter and a photomultiplier tube (PMT) is mounted on a goniometer in a way that it can be moved for scanning the sample area (Fig. 1). For collecting the UV-photons, a 240 mm quartz lens was used. Measurements were done using the UVC-photons and the UVA-photons. For the two different measurements, two different PMTs with different spectral responses were chosen. For the UVC a Hamamatsu H11870-09 with a spectral response between 185 and 320 nm was used. The 254 nm emission peak was selected by a Semrock FF01-260/16–25 filter. The spectral response between 230 and 700 nm was measured with an H10682-210 PMT for UVA and two filters, a Semrock FF01-340/12–25 and an Edmund Optics 337/10, to select photons of the 337 nm emission peak. As reported by Sand et al. [6] the photon count rate strongly depends on the distance between source and detector. Therefore, it was kept constant at 2 m for all measurements and samples. The detection of the radioluminescence signal at distances larger than 2 m will be done in future experiments. At this distance, the UVA-detector has a field of view (FOV) of 19 mm in diameter whereas the UVC-detector has a FOV of 50 mm in diameter. The smaller the FOV, the better is the spatial resolution of the reconstructed image of the scanned source. Therefore, the measured size of the radioluminescence glow in the UVA-scan is much closer to the real size of the glow than the measured size from the UVC-scan.
The optical scanning system
The optical system is mounted at a distance of 2 m away from the sample. Depending on the kind of measurement, the gas chamber can be filled with air or different gas mixtures like N2 and 10 ppm NO. The PMT and corresponding set of filters is changed for measurements in UVA and or UVC
The samples were placed into a measurement chamber with a quartz window, which can be pumped and filled with any gas. The measurements in the UVA region were done in air and in an artificial atmosphere of pure nitrogen with 10 ppm nitric oxide (NO). Measurements in UVC were only made in the artificial atmosphere. The measurement time per pixel depends on the light yield detected from the sample. In the artificial atmosphere, the light yield is expected to be high. Therefore, the time per pixel was set to 0.2 s in UVC and 2 s in UVA (Table 1). The UVA-measurements in air needed a longer measurement time of up to 20 s per pixel because of the missing scintillation effect of NO and the strong quenching of oxygen and water vapour.
Image processing
The recorded radioluminescence signal requires post-processing. Information about sample location and signal intensity is hard to see from the raw data as shown in Fig. 2a. The scan was smoothed by grouping neighbouring pixels, so that the information hidden in the noise becomes available. Figure 2 shows the smoothing process from raw data (a) via 3 pixels smoothing (b) to 9 pixels smoothing (e) which is used for all measurements. In (e) the radioactive source is clearly distinguishable from the background. The signal at the scan edges (Fig. 2c, d, e) originate from reflections of the gas chamber and surrounding objects. The background and the maximum photon count rate can be calculated from the processed scan. The resulting image (Fig. 2e) is described in more detail in Fig. 6f.
Results and discussion
The alpha-track-detection-method shows the distribution of alpha emitters on a sample. Therefore, it is especially suitable for finding small radioactive sources or contamination on a sample, for example finding so-called Hot particles in soil samples [17, 18]. In this work, alpha-track-detection was used for analysing the homogeneity of the alpha-emitter distribution on the pitchblende samples. Samples chosen for radioluminescence measurements should yield a high amount of alpha emitters per area since the optical system is still under development and improvement. Other groups used commercially available sources of several kBq to MBq to detect the radioluminescence signal [2, 14]. Accordingly, it is necessary to start radioluminescence measurements on environmental samples with properties close to standard large area sources. The acquired microscope images from the alpha-track-detectors show black spots representing the track of each alpha particle (Fig. 3a). By adjusting the threshold settings of the software ImageJ the tracks become clearly visible (Fig. 3b).
From the processed image, the number of tracks was counted allowing an estimation of the surface activity. In the example of sample L (Fig. 3) 548 tracks per minute were detected. This representative image is one of 260 images taken from this sample each with a size of 2.49 mm by 1.87 mm. The large image of sample L is shown in Fig. 4 with the edges of the sample marked with a bold line. It shows a homogeneous distribution of tracks except for a small region at the bottom of the sample beneath the thin line. There are only a few tracks detected, indicating waste-rock, not containing uranium. The same applies for sample A above the thin line and sample E outside the thin lines (Fig. 4). All other samples have a homogenous distribution of alpha-emitters over the entire surface area as shown in Fig. 4.
GIC-Spectra of pitchblende samples J, K, L, M and Mix
The measurement time was normalized to 3000 s for each spectrum. They show a large amount of low energy alpha particles due to self-absorption. Numbers identify nuclides of the 238U decay chain whereas letters identify nuclides of the 235U decay chain
Furthermore, alpha-spectroscopy was performed using a grid ionisation chamber (GIC). Since pitchblende is a solid material with homogeneous distribution of uranium inside the bulk, self-absorption of alpha radiation takes place. This self-absorption causes the pronounced low energy tails of alpha particle energy in the spectra of Fig. 5.
Radioluminescence scans
The measurement in UVC yields the highest intensity for all samples (upper row) and the biggest radioluminescence glow. Both measurements in UVA have a smaller glow due to the smaller field of view. The background rate is higher as for measurements in UVC. Using the artificial atmosphere (middle row) leads to a three times higher signal than measuring in air (lower row). All samples have a similar surface activity and a similar photon count rate.
Uranium and its solid daughters are identified by their maximum alpha energy edges. Their peaks are not as narrow as for example the 222Rn peak due to the absorption process. Radon as a noble gas is able to leave the solid material and decreases self-absorption. Additionally, the efficiency for detection of a radioactive gas in a gas chamber is twice as high as for a solid surface sample. Therefore, radon is represented as peaks in the spectra. Radon daughters show a mixed behaviour as they may contribute from the solid material with self-absorption or from the gas without absorption. Each peak belongs to a nuclide of a decay chain of uranium-238 or uranium-235.
From these spectra, the surface count rate of the sample could be estimated. Since only alpha particles emitted from the surface into the upper hemisphere were detectable, the calculated surface count rate is maximum half of the total surface activity of the sample. The results of this calculation are shown in Table 2. All samples have a detectable alpha count rate between 1 kBq and 1.5 kBq.
Although having a high impact on the measurements with the GIC, the influence of radon on radioluminescence measurements is negligible. The small amount of emerging gas is directly diluted in the gas chamber. Its alpha particles contribute to the total radioluminescence signal but little compared to the signal produced by the alpha particles of the solid pitchblende sample. The samples were in the chamber during the measurements, which lasted a maximum of eleven hours for the longest measurement. This time was not long enough to accumulate radon in the chamber. The chamber was flushed, between different samples and measurements
Three different radioluminescence measurements were performed for three different pitchblende samples to investigate their influence on the count rate depending on the wavelength and the measurement atmosphere. The variation of the surface count rate is very small. Thus, the samples were expected to generate similar photon count rates under the same measurement conditions
Since the surface activity of the pitchblende samples is low, the first radioluminescence measurements were done in the N2 + 10 ppm NO atmosphere for maximum sensitivity. For both wavelengths, UV-photons were measurable and the radioactive source is clearly distinguishable from the background. Measurements of UVC in air were not performed because the UVC light yield in air is too low to be detected against the background. The detection of UVA in air was possible but required a much longer acquisition time per pixel, because the signal-to-background-ratio is below 0.4 (Table 3). This is due to the large quenching of the excited nitrogen by oxygen and water vapour in air
Nevertheless, these measurements of UVA in air are very important, because the optical detection systems are to be used in the environment where air is the only available medium and the control of the atmospheric conditions is not possible
The measurements of the 254 nm wavelength (UVC) in the artificial atmosphere have the lowest background rate of 1.5 ± 0.7 photons per second and pixel on average. Additionally, the maximum photon count rate is between 126 ± 33 and 163 ± 38 photons per second and pixel (Table 4). This leads to a signal-to-background-ratio up to 50 times higher than in the comparable measurements in UVA. As mentioned before, the presence of small amounts of NO shifts the emission spectrum of the gas towards UVC, since NO emits mainly at wavelengths shorter than 300 nm [10]
The background for the measurements of 337 nm is around 3.5 ± 0.2 photons per second and pixel. It is about twice the background at 254 nm, due to a higher contribution of scattered photons from lamps and electronics in this spectral band as well as a higher background of the UVA-PMT. In pure nitrogen the UVA-signal is increased by at least a factor of six compared to ambient air. It is in perfect agreement to the results of Sand et al. [6]
The photon count rates for the different samples are relatively similar as they have approximately the same surface activity. However, their surface areas are very different and range from 6.6 cm2 (sample L) to 18.1 cm2 (sample Mix). The difference in sample size leads to a variation in activity per cm2. Sample L as the smallest sample has the highest density of alpha-emitters of 156 cm-2, whereas sample Mix has only 83 cm-2. This results in a higher photon count rate per pixel for sample L although its surface activity is smaller than the one of sample Mix. For all samples, the highest count rate was over 100 counts per second and pixel measured in UVC. In comparison to UVC, the UVA measurements have a significant lower maximum photon count rate, but show the same behaviour with sample Mix as the one with the lowest count rate. The different surface areas are not only visible in the intensity but also in the size of the radioluminescence glow (Fig. 6). The more extended the source the larger the glow. It is also larger for the UVC measurements since the field of view of the UVC-detector is bigger than the field of view of the UVA-detector. Therefore, the UVC-signal is nearly circular with the highest intensity in the middle of the radioluminescence glow and contains no more information about the geometry of the samples. In Fig. 6b, the UVA-signal in the N2 + NO atmosphere is shown. The scan reveals information about the location of the highest density of alpha-emitters on the sample. This is in contrast to Fig. 3a where the image of the same sample is shown but made using the UVC-PMT with its larger FOV. This scan contains only information about the location of the source not about its alpha-emitter distribution
The low signal-to-background-ratio (Table 3) leads to blurring of the UVA scans (Fig. 6). Higher photon count rates at the scan edges (Fig. 6f) do not indicate presence of alpha emitters but rather originate from UVA-photon-reflections of surrounding objects. However, in spite of these artefacts, the location of the pitchblende samples is clearly identifiable from the radioluminescence signal in all cases.
The results of the different alpha detection methods are compared in Table 5.
The photon count rates for one kind of measurement show a variation between the three samples of one order of magnitude or less. Due do the missing scintillation effect of nitric oxide, the measurement in air has not only the lowest maximum count rate but self-evidently also the lowest total amount of radioluminescence photons per second. The order of the samples from highest to lowest within one kind of measurement is different for the maximum photon count rate and the total count rate for all measurements. There are two possible reasons for that. First, the samples have a very similar surface activity, which leads to an expected similar photon count rate for all samples. Second, pitchblende is a naturally occurring mineral with a possibly heterogeneous distribution of alpha emitters. Areas with a high density of alpha emitters increase the maximum count rate but may not affect the total count rate. Additionally, the large background in the UVA spectral range, almost as large as the maximal count rate, leads to a greater uncertainty in background corrected total photon count rate
Finally, an experiment under realistic conditions was performed. All samples were placed on a total area of 30 by 40 cm and arranged at equal distance from each other, for measurements in the UVA spectral range in air. The measurement took 64 h, which is an admittedly very long time span for a proposed remote sensing method. These first attempts, however serves as proof of principle and as a starting point for further improvements. The locations and even shapes of the pitchblende samples were well recognizable on the scan (Fig. 7). The background-corrected maximum photon count rate is 1.4 photons per pixel and second. Some reflections from background photons disturb the bottom of the scan but had no influence on locating the samples. With this experiment, we were able to prove the feasibility of measuring environmental samples of low activity in air in the UVA spectral range
Conclusion
The aim of this study was to proof the feasibility of detecting low activity in environmental samples by measuring their radioluminescence with the optical detection system. Before the radioluminescence was measured, all samples were characterized with conventional alpha detection methods. Their surface activity was determined from their GIC-spectra and ranges from 80 Bq cm-2 to 105 Bq cm-2. The optical system was used for two different wavelengths, 254 nm in the UVC-band of the electromagnetic spectrum and 337 nm in the UVA-band. The maximum count rate per pixel obtained in the artificial atmosphere was about 50 times higher in UVC than in UVA, due to a difference in the scintillation strength of nitric oxide for the two wavelengths. The use of an artificial atmosphere of nitrogen with 10 ppm nitric oxide provided a significant increase of the radioluminescence signal for all pitchblende samples compared to air.
Depending on the intensity of the radioluminescence, the acquisition time per pixel varied for the different measurements but was consistent for all samples within one measurement. Measurements in air needed the longest acquisition time per pixel (10 to 20 s), which directly effects the measurement duration. The scanning of all samples in air needed 64 h for an area of 30 by 40 cm.
In this work, pitchblende samples of a low specific alpha activity were measured from a distance of 2 m. Since the optical detection system developed by RemoteALPHA is meant to be used for radiological emergencies with very high alpha contaminations, our results show that it is possible to detect them. High alpha activities will lower the acquisition time per pixel, which will speed up the measurements. Compared to conventional time-consuming measurements by hand, the optical detection system will scan an area much faster, while it will not expose its operators to the risk of ionizing radiation or potentially hazardous materials, fire and debris.
References
Yao J, Brenizer J, Hui R, Yin S (2013) Standoff alpha radiation detection via excited state absorption of air. Appl Phys Lett 102:1–6
Sand J et al (2016) Stand-Off Radioluminescence Mapping of Alpha Emitters under Bright Lighting. IEEE Trans Nucl Sci 63:1777–1783
Crompton AJ et al (2018) Gas flow to enhance the detection of alpha-induced air radioluminescence based on a UVTron flame sensor. Sens (Switzerland) 18:1–11
Sand J et al (2015) Imaging of alpha emitters in a field environment. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip 782:13–19
Brett J et al (2017) Spectral measurements of alpha-induced radioluminescence in various gases. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip 874:88–93
Sand J, Ihantola S, Peräjärvi K, Toivonen H, Toivonen J(2014) Radioluminescence yield of alpha particles in air.New J. Phys.16,
Dandl T, Heindl T, Ulrich A(2012) Fluorescence of nitrogen and air.J. Instrum.7,
Johan Sand Alpha Radiation Detection via Radioluminescence of Air.
Ivanov OP, Stepanov VE, Smirnov SV, Volkovich AG (2011) Development of method for detection of alpha contamination with using UV-camera ‘dayCor’ by OFIL. IEEE Nucl. Sci. Symp. Conf. Rec. 2192–2194 doi:https://doi.org/10.1109/NSSMIC.2011.6154448
Kerst T, Toivonen J (2018) Intense radioluminescence of NO/N 2 -mixture in solar blind spectral region. Opt Express 26:33764
Waldenmaier T, Blümer J, Klages H (2008) Spectral resolved measurement of the nitrogen fluorescence emissions in air induced by electrons. Astropart Phys 29:205–222
Krasniqi FS et al (2021) Standoff UV-C imaging of alpha particle emitters. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip 987:164821
EC. Recommendation from the Scientific Committee on Occupational Exposure Limits for benzene. 1–28 (1991)
Crompton AJ, Gamage KAA, Jenkins A, Taylor CJ(2018) Alpha particle detection using alpha-induced air radioluminescence: A review and future prospects for preliminary radiological characterisation for nuclear facilities decommissioning.Sensors (Switzerland)18,
Baschenko SM (2004) Remote optical detection of alpha particle sources. J Radiol Prot 24:75–82
Giakos GC (2008) Stand-off detection of mixed radiation fields. Heal Monit Struct Biol Syst 2008 6935:69351F
Bondarenko OA, Salmon PL, Henshaw DL, Fews AP, Ross AN (1996) Alpha-particle spectroscopy with TASTRAK (CR-39 type) plastic, and its application to the measurement of hot particles. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip 369:582–587
Hamann L(2017) Investigation on radioactive particles via secondary ion mass spectroscopy. PhD thesis
Acknowledgements
The project 19ENV02 RemoteALPHA has received funding from the EMPIR programme co-financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme. 19ENV02 RemoteALPHA denotes the EMPIR project reference. The authors would like to acknowledge Dr. Thomas Hassel from the institute of material science in Hanover for cutting the pitchblende stones into slices. Julia Stadler for editing the manuscript and Anna Kogiomtzidis for writing a program for data analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest do declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klose, A., Luchkov, M., Dangendorf, V. et al. On the way to remote sensing of alpha radiation: radioluminescence of pitchblende samples. J Radioanal Nucl Chem 331, 5401–5410 (2022). https://doi.org/10.1007/s10967-022-08540-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08540-6