Abstract
Wild boars (Sus scrofa) are notorious for accumulating high contamination levels of 137Cs in their meat. Publicly available data of 137Cs contamination levels in wild boars from 2011 to 2019 were used to determine some radioecological characteristics in Germany (affected by Chernobyl-fallout, 1986) and Japan (affected Fukushima, 2011). The effective half-life of 137Cs in wild boar meat was much longer in Germany (7.3 y) than in Japan (2.6 y), respectively. Wild boars in Germany thus show much more persistent contamination levels than other game or forest animals. This unusual behavior has been termed “wild boar paradox.” In German wild boars, the data sets reveal a distinct geographical and seasonal dependence with higher activity concentrations in winter than in summer. In Japan, contamination levels only exhibit a distinct decline behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nuclear accidents at Chernobyl (1986) and Fukushima (2011) have caused widespread contamination on a global and national level, respectively. Following major nuclear accidents, protection of the public receives major attention, in particular food safety. In the immediate aftermath of a nuclear accident, it has been shown previously [1,2,3,4,5] that the ingestion of radionuclides with contaminated food is the main additional dose contributor for the public. Of all radionuclides emitted, radioactive cesium, in particular 134Cs (physical half-life T1/2 = 2.1 y) and 137Cs (T1/2 = 30.1 y), plays an important role due to its volatility and high specific activity and persistence in the environment as a result of the long half-life of 137Cs. The contaminations from Chernobyl and Fukushima, respectively, thus remain relevant for many decades to come. To a lower extent, fallout from nuclear weapons testing may contribute to the total radiocesium. For Europe, this impact, however, is in the range of only about 10% of the total radiocesium in soil [6]. Fallout from Chernobyl and Fukushima, respectively, thus will dominate the contamination levels in the ecosystems in Germany and Japan, respectively.
Wild game has been identified as a potent accumulator of radiocesium [7,8,9], in particular wild boar [7, 8, 10,11,12,13,14,15,16,17,18,19,20]. Whereas most forest species have exhibited high contamination levels in the early aftermath follwed by decline, strangely, a similar behavior in the dynamics of the contamination could not be observed for wild boars. Wild boars have been shown not only to accumulate radiocesium to a high extent, but also to largely maintain these contamination levels over the periods of years or decades [7, 8, 12, 18, 21, 22].
The continuous intake of radiocesium and the preference for cesium-rich food seems to be responsible for this unique characteristic in wild boars. Especially, the mushroom diet of wild boars is a likely key factor in the contamination of wild boars due to the mushrooms’ cesium-accumulating characteristics [23,24,24,25,26,27,28,30,31]. Hypogeous fungi such as deer truffle (Elaphomyces) have been suggested as the major source of radiocesium in the soft tissue of wild boars [18, 32], rather than any physiological anomaly of the wild boar (such as the accumulation in fat tissue [14, 33]). This anomalous behavior warranted for an in-depth analysis of the temporal and spatial contamination dynamics of 137Cs in wild boar and to elucidate the impact of environmental factors such as the availability of less contaminated foods as well as the impact of the density of forestation. In order to assess these different aspects, the dense and publicly available environmental monitoring data compiled in Germany and Japan, respectively, were harnessed in this study.
Data and methods
For Germany, data sets of the 137Cs activity concentration in wild boars, compiled by the German Federal Office for Radiation Protection (Bundesamt für Strahlenschutz, BfS) were used, which include samples from January 5, 2012 to March 13, 2019. While data from the last three years is made publicly available [34], older data were provided upon request. The Japanese food data was collected by the Ministry of Health, Labor and Welfare [35], with sampling dates of 137Cs, 134Cs and total radiocesium (134Cs + 137Cs) activity concentrations in wild boars ranging from May 8, 2011 to July 17, 2018. In cases, where the 137Cs activity concentration was not reported, it was calculated from the total radiocesium activity concentration as described in [17]. Only the 137Cs activity concentration was used in the analyses presented here in order to enable a comparison with the German food data. Measurements reported being lower than the detection limit were treated as having a value of half the detection limit in both data sets. Due to the great variance of the measurements, a lognormal distribution was assumed and the geometric mean was considered more representative than the arithmetic mean for most statistical analyses.
Results and discussion
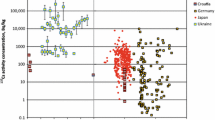
Cesium-137 activity concentrations in boars show a large variability throughout all years of observation with values ranging from less than 10 Bq/kg to more than 10 000 Bq/kg.
Spatial distribution
Sampling locations are not uniformly spread throughout the countries, with significant ”gaps” in-between (locations from where no samples were reported) (Fig. 1). The figure shows the activity concentrations (geometric means) in the meat of wild boars per municipality (at least one boar per municipality). Geometric means were also used by other authors, e.g., Fuma et al. [10], and were found to reflect the dynamic characteristics best. In municipalities indicated in gray in Fig. 1, no meat sample was delivered for measurement or measurements were not included in the database. Locations also varied with each year as shown in Fig. 2, making it difficult to assess the combined temporal and spatial distribution of contamination in wild boars in detail. The spatial pattern shown in Fig. 1, therefore, should be interpreted as an overview of “hot spots” only. Especially high activity concentrations can be observed in Northern Bavaria, Germany, correlating with the spread of radionuclides from the Chernobyl nuclear accident in 1986 [36]. Likewise, measurements of high activity concentrations in the Japanese prefectures of Fukushima and Ibaraki roughly match radionuclide depositions reported after the Fukushima nuclear accident [37, 38].
Samples in Bavaria are the most uniformly distributed of the German data set, allowing best for comparison of 137Cs contamination in boars and land usage in the corresponding area. Data for boars in Germany imply that high levels of contamination are positively correlated with the degree of forestation [39] (Fig. 2, Germany – bottom right). Obviously, the nutrition for boars in forested areas includes a greater load of 137Cs than for wild boars that supplement their diet with agricultural products for human consuption (e.g., corn).
Spatial distribution of boars’ 137Cs contamination by year (left: Germany; right: Japan). For each year, the geometric mean of a municipality is shown. In the lower right corner of the left figure, a map that shows the forest coverage of Germany is displayed (taken from ref. [39])
Effective and ecological half-lives
The observed declining behavior of radionuclides in plants and animals is calculated by the effective half-life
Here, Tphys is the physical half-life, and Tecol is the ecological half-life, describing the loss of activity in an ecosystem by dilution, excretion or migration (in soil) mechanisms. In Fig. 3, the effective half-life of 137Cs contamination in wild boars was computed for Germany and Japan respectively. For Germany, a value of 7.3 years was obtained, whereas in Japan the effective half-life amounts to 2.6 years. Note that due to low numbers of samples in each municipality, we had to pool the samples for each country. Here some uncertainty is introduced by some fluctuations in the sample numbers provided each year from municipalities with higher or lower contamination levels. Also note that the effective half-lives in game animals may fluctuate significantly [40]. According to the above equation, the ecological half-lives of 137Cs in wild boars are 10.2 y for Germany and 2.8 y for Japan, respectively. There are several factors that affect the ecological half-life of radiocesium in a species. Differences have been observed between species and climates. For example, roe deer from central Europe was shown to exhibit ecological half-lives between 7–9 y [7], whereas white-tailed deer from the Savannah River Site exhibited much longer ecological-half-lives of 23.15 y [41]. For wild boar, the variability is even more pronounced. Previous studies found 10.5±1.6 y in Germany [42] or even 62 y [43] or 78 y [44], which leads to an increase rather than a decline of contamination levels (i.e. essentially the ”wild boar paradox”). Most recently, an ecological half-life of 16.49 was published for north-east Poland [40].
In any case, the downward trend in activity concentration is more pronounced for Japanese wild boars than for German wild boars. This result is consistent with the aforementioned influence on radiocesium contamination of deer truffles in the boars’ diet, although there could be other co-founding factors such as forestation or availability of alternative food sources (which again would relate to the impact of deer truffles). Unfortunately, deer truffles are difficult to find, which explains why they largely remain an understudied factor in radioecology. Therefore, it is difficult to test the key hypothesis of the paradox directly.
All data points for 137Cs in meat of wild boars in Germany (A) and Japan (B) by their sampling date. The color specifies the respective state or prefecture (only areas with the highest sample sizes are marked). The red line is an exponential fit of the entity of data; the obtained parameters and coefficient of determination are given in the box in the lower right corner of each plot
The most common species of deer truffles, Elaphomyces granulatus, has been described as widespread and common throughout Germany [32], but they are rare in Japan [45]. The slower decline dynamics of radiocesium in German wild boars (in contrast to Japan) may be caused by the boars’ specific diet and hence may explain our finding of a much longer effective half-life in Germany.
Decline of 137Cs activity concentrations varies a lot by location (Fig. 4). Especially areas with a higher degree of contamination such as Bavaria, Germany or Fukushima prefecture, Japan show a faster decline than lesser contaminated ones. Some areas even show a slight increase of activity concentrations, which could possibly be attributed to regionally varying diets of wild boars, leading to differences in radiocesium intake. Similar effects have already been observed in other studies [7, 18].
Seasonal variations
The data shows substantial seasonal variations over the course of a year in Germany (Fig. 5, top), while in Japan, although fluctuating as well, activity concentrations do not show a noticeable seasonality (Fig. 5, bottom). In any case, a distinct general decline behavior of the contamination levels is obvious for Japanese wild boar, but not nearly as pronounced for German wild boar.
Seasonal variation of 137Cs contamination in wild boars in Germany (top) and Japan (bottom). The geometric mean for each month of the sampling period is plotted at the respective month’s start date. Error bars represent the geometric standard error. Due to the y-axis being logarithmically scaled, most of the lower bounds are not visible. Dashed lines only guide the eye and have no further implication
In Germany, activity concentrations tend to be higher in winter and lower in summer in most years. Agricultural products and tree seeds compose a large portion of the wild boars’ diet[21]. Availability of these foods falls largely into the late summer months and fall. During winter and early spring, boars are often forced to search for underground food sources like the aforementioned, highly radiocesium-accumulating Elaphomyces fungi. This explanation thus is consistent with our observations for the situation in Germany. In Japan, the situation seems to be different as the seasonal fluctuation of the 137Cs concentration in boars seems to be much less pronounced. This observation is in line with a previous study that found no distinct correlation between the food items in the stomachs of Japanese wild boar and the contamination level of the respective boar meat [46]. The reason may be that there is no such single food item in Japan that dominates the boars’ contamination like the deer truffle in Germany.
The influence of ‘mast years’ on activity levels in wild boars is another interesting aspect worth of consideration. Mast years are sporadically recurring years with abnormally high production of tree seeds (primarily acorns and beechnuts). These seeds constitute an important fraction of the boars’ diet. In Germany, the years 2012, 2016, and 2018 have been classified as mast years in the literature [47]. Figure 6 shows that the arithmetic mean calculated for October of these three years is lower than in the others, albeit not statistically significant (p = 0.05), as revealed by a Tukey-Kramer test. This distinct feature is also only observable when the arithmetic mean is applied to the data in contrast to the geometric mean that is used throughout the rest of this study (as well as in most of the literature that is available on wild boar contamination). In October, which is the ripening month of acorns and beechnuts, the availability of these seeds reaches a peak and saves the boars the effort of digging for underground fungi. Both acorns and beechnuts are notorious for low radiocesium levels. For example, a 2001 study showed contamination levels as low as 6.5±0.1 Bq 137Cs per kg for acorns from Croatia [48]. For beechnuts, no literature data are available, but since beech wood exhibits similar contamination levels like oak wood, beechnuts are unlikely at any higher levels than acorns [48]. In contrast, the radiocesium levels in deer truffels are significantly higher, reaching 5,000 Bq∙kg− 1 [49]or even 25,000 Bq∙kg− 1 [50]. In Japan, as discussed previously, mushrooms and underground foods are no dominant contributors to the wild boars’ contamination levels [46].
Of course, a mast year would not result in a sudden drop of already existing radiocesium contaminations in the meat, but a decline according to the biological half-life (excretion of radiocesium). However, sudden availability of plenty of low-contaminated, easily accessible fodder would immediately cease the intake of highly contaminated underground food sources. We thus may expect a delayed effect over several months rather than a step function. The seasonal zig-zag pattern for seasonal contamination levels in Fig. 5 is certainly more pronounced in Germany than in Japan.
Comparison on an international scale shows that radiocesium levels in wild boars are in the same range in Poland [40] like in Germany (however, it appears that fewer specimen reach the 104 Bq∙kg− 1 range). Polish wild boars also show a distinct ”wild boar paradox.” In Southern Italy, much less affected by Chernobyl fallout, levels are about two orders of magnitude lower than in Germany or Japan [51].
Conclusion
Radiocesium contaminations in wild boars in Germany and Japan are in line with radionuclide depositions after nuclear accidents. A decline in activity is clearly visible in Japan, while in Germany, activity concentration levels remain rather constant even many years after the Chernobyl nuclear accident. These unique decline dynamics could be caused by a fungus in the wild boars’ diet, the radiocesium accumulating deer truffle. In Germany, the contamination shows a distinct dependency on seasons, with generally lower activity concentrations in summer and autumn, indicating a notable influence of seasonally available food sources on contamination. Greater availability of tree seeds in mast years is noticeable in lower activity concentration levels in the month of October but no level of statistical significance was reached for these effects.
Change history
10 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10967-022-08628-z
References
Hamada N, Ogino H (2012) Food safety regulations: what we learned from the Fukushima nuclear accident. J Environ Radioact 111:83–99. doi:https://doi.org/10.1016/j.jenvrad.2011.08.008
Hamada N, Ogino H, Fujimichi Y (2012) Safety regulations of food and water implemented in the first year following the Fukushima nuclear accident. J Radiat Res 53(5):641–671
Steinhauser G, Chávez-Ortega M, Vahlbruch J-W (2017) Japanese food data challenge the claimed link between Fukushima’s releases and recently observed thyroid cancer increase in Japan. Sci Rep 7(1):10722. doi:https://doi.org/10.1038/s41598-017-10584-8
Travnikova IG, Bruk GJ, Shutov VN, Bazjukin AB, Balonov MI, Rahola T, Tillander M (2001) Contribution of different foodstuffs to the internal exposure of rural inhabitants in Russia after the Chernobyl accident. Radiat Prot Dosimetry 93(4):331–339. doi:https://doi.org/10.1093/oxfordjournals.rpd.a006445
Mueck K, Suda M, Gerzabek M, Kunsch B (1992) Ingestion Dose Response to the Deposition Date in the First Year after Radionuclide Deposition. Radiat Prot Dosimetry 42(2):103–114. doi:https://doi.org/10.1093/oxfordjournals.rpd.a081285
Bossew P, Falkner T, Henrich E, Kienzl K (1995) Cäsiumbelastung der Böden Österreichs. Umweltbundesamt, Wien
Strebl F, Tataruch F (2007) Time trends (1986–2003) of radiocesium transfer to roe deer and wild boar in two Austrian forest regions. J Environ Radioact 98(1–2):137–152. doi:https://doi.org/10.1016/j.jenvrad.2006.02.009
Tataruch F, Schönhofer F, Onderscheka K (1988) Untersuchungen zur radioaktiven Belastung der Wildtiere in Österreich. Z Jagdwiss 34:22–35 (in German)
Potter CM, Brisbin IL, McDowell SG, Whicker FW (1989) Distribution of 137Cs in the American Coot (Fulica americana). J Environ Radioact 9(2):105–115. doi:https://doi.org/10.1016/0265-931X(89)90018-0
Fuma S, Kubota Y, Ihara S, Takahashi H, Watanabe Y, Aono T, Soeda H, Yoshida S (2016) Radiocaesium contamination of wild boars in Fukushima and surrounding regions after the Fukushima nuclear accident. J Environ Radioact 164:60–64. doi:https://doi.org/10.1016/j.jenvrad.2016.07.002
Anderson D, Okuda K, Hess A, Nanba K, Johnson T, Takase T, Hinton T (2019) A comparison of methods to derive aggregated transfer factors using wild boar data from the Fukushima Prefecture. J Environ Radioact 197:101–108. doi:https://doi.org/10.1016/j.jenvrad.2018.12.009
Gulakov AV (2014) Accumulation and distribution of 137Cs and 90Sr in the body of the wild boar (Sus scrofa) found on the territory with radioactive contamination. J Environ Radioact 127(0):171–175. doi:https://doi.org/10.1016/j.jenvrad.2013.06.008
Nihei N, Tanoi K, Nakanishi TM (2016) Monitoring inspection for radiocesium in agricultural, livestock, forestry and fishery products in Fukushima prefecture. J Radioanal Nucl Chem 307(3):2217–2220. doi:https://doi.org/10.1007/s10967-015-4448-z
Tanoi K, Uchida K, Doi C, Nihei N, Hirose A, Kobayashi NI, Sugita R, Nobori T, Nakanishi TM, Kanno M, Wakabayashi I, Ogawa M, Tao Y (2015) Investigation of radiocesium distribution in organs of wild boar grown in Iitate, Fukushima after the Fukushima Daiichi nuclear power plant accident. J Radioanal Nucl Chem 307(1):741–746. doi:https://doi.org/10.1007/s10967-015-4233-z
Sontag G, Weinke HH, Scholz H (1989) Radioaktivität in Wildfleisch (Radioactivity of game meat). Ernährung/Nutrition 13(8/9):494–499 (in German)
Saito R, Nemoto Y, Tsukada H (2020) Relationship between radiocaesium in muscle and physicochemical fractions of radiocaesium in the stomach of wild boar. Sci Rep 10(1):6796. doi:https://doi.org/10.1038/s41598-020-63507-5
Steinhauser G, Saey PRJ (2016) Cesium-137 in the meat of wild boars: a comparison of the impacts of Chernobyl and Fukushima. J Radioanal Nucl Chem 307:1801–1806. doi:https://doi.org/10.1007/s10967-015-4417-6
Semizhon T, Putyrskaya V, Zibold G, Klemt E (2009) Time-dependency of the 137Cs contamination of wild boar from a region in Southern Germany in the years 1998 to 2008. J Environ Radioact 100(11):988–992. doi:https://doi.org/10.1016/j.jenvrad.2009.06.023
Anderson D, Kaneko S, Harshman A, Okuda K, Takagi T, Chinn S, Beasley JC, Nanba K, Ishiniwa H, Hinton TG (2022) Radiocesium accumulation and germline mutations in chronically exposed wild boar from Fukushima, with radiation doses to human consumers of contaminated meat. Environ Pollut 306:119359. doi:https://doi.org/10.1016/j.envpol.2022.119359
Tagami K, Howard BJ, Uchida S (2016) The Time-Dependent Transfer Factor of Radiocesium from Soil to Game Animals in Japan after the Fukushima Dai-ichi Nuclear Accident. Environ Sci Technol 50 (17):9424-9431. doi: https://doi.org/10.1021/acs.est.6b03011
Fielitz U, Richter K (2013) Bundesweiter Überblick über die Radiocäsiumkontamination von Wildschweinen (in German). Vorhaben 3607S04561. Bundesamt für Strahelnschutz
Pohlschmidt J (1996) Untersuchungen zur Radiocäsiumbelastung von Schwarzwild. Staatliches Landesveterinäramt, Hannover
Zhdanova NN, Zakharchenko VA, Haselwandter K (2005) Radionuclides and Fungal Communities. In: Dighton J, White JF, Oudemans P (eds) The Fungal Community, 3rd edn. CRC Press, Boca Raton, pp 759–768
Haselwandter K (1978) Accumulation of the radioactive nuclide cesium-137 in fruitbodies of Basidiomycetes. Health Phys 34:713–715
Prand-Stritzko B, Steinhauser G (2018) Characteristics of radiocesium contaminations in mushrooms after the Fukushima nuclear accident: evaluation of the food monitoring data from March 2011 to March 2016. Environ Sci Pollut Res 25(3):2409–2416. doi:https://doi.org/10.1007/s11356-017-0538-5
Falandysz J, Zalewska T, Krasińska G, Apanel A, Wang Y, Pankavec S (2015) Evaluation of the radioactive contamination in fungi genus Boletus in the region of Europe and Yunnan Province in China. Appl Microbiol Microtechnol 99(19):8217–8224. doi:https://doi.org/10.1007/s00253-015-6668-0
Falandysz J, Zalewska T, Apanel A, Drewnowska M, Kluza K (2016) Evaluation of the activity concentrations of 137Cs and 40K in some Chanterelle mushrooms from Poland and China. Environ Sci Pollut Res 23(19):20039–20048. doi:https://doi.org/10.1007/s11356-016-7205-0
Zalewska T, Cocchi L, Falandysz J (2016) Radiocaesium in Cortinarius spp. mushrooms in the regions of the Reggio Emilia in Italy and Pomerania in Poland. Environ Sci Pollut Res 23(22):23169–23174. doi:https://doi.org/10.1007/s11356-016-7541-0
Falandysz J, Zhang J, Zalewska T, Apanel A, Wang Y, Wiejak A (2015) Distribution and possible dietary intake of radioactive 137Cs, 40K and 226Ra with the pantropical mushroom Macrocybe gigantea in SW China. J Environ Sci Health Part A: Toxic/Hazard Subst Environ Eng 50(9):941–945. doi:https://doi.org/10.1080/10934529.2015.1030289
Guillén J, Baeza A (2014) Radioactivity in mushrooms: A health hazard? Food Chem 154:14–25. doi:https://doi.org/10.1016/j.foodchem.2013.12.083
Falandysz J, Zalewska T, Saniewski M, Fernandes AR (2021) An evaluation of the occurrence and trends in 137Cs and 40K radioactivity in King Bolete Boletus edulis mushrooms in Poland during 1995–2019. Environ Sci Pollut Res 28(25):32405–32415. doi:https://doi.org/10.1007/s11356-021-12433-8
Konopleva I, Klemt E, Konoplev A, Zibold G (2009) Migration and bioavailability of 137Cs in forest soil of southern Germany. J Environ Radioact 100(4):315–321. doi:https://doi.org/10.1016/j.jenvrad.2008.12.010
Steinhauser G, Knecht C, Sipos W (2017) Fat tissue is not a reservoir for radiocesium in wild boars. J Radioanal Nucl Chem 312(3):705–709. doi:https://doi.org/10.1007/s10967-017-5257-3
Bayerisches Landesamt für Umwelt (LFU) (2019) Überwachung der allgemeinen Umweltradioaktivität in Bayern (StrVG). http://www.lfu.bayern.de/strahlung/umrei/strvgprobe. Accessed August 2019
Ministry of Health Labour and Welfare (MHLW) (2019) Levels of Radioactive Contaminants in Foods Tested in Respective Prefectures. http://www.mhlw.go.jp/english/topics/2011 eq/index_food_radioactive.html. Accessed June 2019
Rosner G, Winkler R (2001) Long-term variation (1986–1998) of post-Chernobyl 90Sr, 137Cs, 238Pu and 239,240Pu concentrations in air, depositions to ground, resuspension factors and resuspension rates in south Germany. Sci Total Environ 273(1):11–25. doi:https://doi.org/10.1016/S0048-9697(00)00716-6
Kinoshita N, Sueki K, Sasa K, Kitagawa J-I, Ikarashi S, Nishimura T, Wong Y-S, Satou Y, Handa K, Takahashi T, Sato M, Yamagata T (2011) Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc Natl Acad Sci USA 108(49):19526–19529. doi:https://doi.org/10.1073/pnas.1111724108
Rosenberg BL, Ball JE, Shozugawa K, Korschinek G, Hori M, Nanba K, Johnson TE, Brandl A, Steinhauser G (2017) Radionuclide pollution inside the Fukushima Daiichi exclusion zone, part 1: Depth profiles of radiocesium and strontium-90 in soil. Appl Geochem 85:201–208. doi:https://doi.org/10.1016/j.apgeochem.2017.06.003
Bundeswaldinventur(2019) Anteil der Fläche an der Gesamtfläche Wald + Nichtwald [%] nach Waldspezifikation (in German)
Oloś G, Dołhańczuk-Śródka A (2022) Effective and environmental half-lives of radiocesium in game from Poland. J Environ Radioact 248:106870. doi:https://doi.org/10.1016/j.jenvrad.2022.106870
Gaines KF, Novak PM, Novak JM (2021) Ecological half-life of radiocesium in white-tailed deer on the Department of Energy’s Savannah River Site: What can a half century of field monitoring tell us? J Environ Radioact 235–236:106654. doi:https://doi.org/10.1016/j.jenvrad.2021.106654
Zibold G, Klemt E (2005) Ecological half-times of 137Cs and 90Sr in forest and freshwater ecosystems. Radioprotection 40:S497–S502
Hecht H (1997) Radiocäsiumverteilung und -trends bei Wildbret in Bayern. Abschlussbericht zum Forschungsvorhaben im Auftrag des Bayerischen Staatsministeriums für Landesentwicklung und Umweltfragen. Bundesanstalt für Fleischforschung, Kulmbach (Radiocaesium Distribution and Trends in Game Meat of Bavaria). Final Report Federal Institute for Meat Research Kulmbach:(in German
Fielitz U(2005) Untersuchungen zum Verhalten von Radiocäsium in Wildschweinen und anderen Biomedien des Waldes. Final Report for StSch 4324:(in German)
Iwabuchi S, Sakai S, Yamaguchi O (1994) Analysis of mushroom diversity in successional young forests and equilibrium evergreen broad-leaved forests. Mycoscience 35(1):1–14. doi:https://doi.org/10.1007/BF02268522
Nemoto Y, Oomachi H, Saito R, Kumada R, Sasaki M, Takatsuki S (2020) Effects of 137Cs contamination after the TEPCO Fukushima Dai-ichi Nuclear Power Station accident on food and habitat of wild boar in Fukushima Prefecture. J Environ Radioact 225:106342. doi:https://doi.org/10.1016/j.jenvrad.2020.106342
NABU Baden Württemberg (2016) Mastjahr 2016: Buche verwandelt Wald erneut in ein Schlaraffenland. https://baden-wuerttemberg.nabu.de/news/2016/21157.html. Accessed June 2019
Hus M, Košutić K, Lulić S (2001) Radioactive contamination of wood and its products. J Environ Radioact 55(2):179–186. doi:https://doi.org/10.1016/S0265-931X(00)00191-0
Dvořák P, Snášel P, Beňová K (2010) Transfer of Radiocesium into Wild Boar Meat. Acta Vet Brno 79. doi:https://doi.org/10.2754/avb201079S9S085
Steiner M, Fielitz U (2009) Deer truffles – the dominant source of radiocaesium contamination of wild boar. Radioprotection 44(5):585–588
Caridi F, D’Agostino M, Belvedere A (2020) Radioactivity in Calabrian (Southern Italy) Wild Boar Meat. Appl Sci 10(10):3580
Acknowledgements
We thank Martin Steiner for providing access to the data set from Germany and for interesting and helpful discussions. We thank Bayerische Akademie für Jagd und Natur for their generous support of our wild-boar-related work.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berendes, O., Steinhauser, G. Exemplifying the “wild boar paradox”: dynamics of cesium-137 contaminations in wild boars in Germany and Japan. J Radioanal Nucl Chem 331, 5003–5012 (2022). https://doi.org/10.1007/s10967-022-08528-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08528-2