Abstract
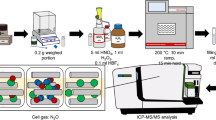
A direct mercury analyzer (DMA) was used to analyze total mercury in radioactive waste samples containing methylmercury and other forms of mercury including Hgo (elemental) together with inorganic ionic mercury species and complexes. These samples were also analyzed using Cold Vapor Atomic Absorption Spectroscopy (CVAAS) and/or Inductively Coupled Plasma Mass Spectroscopy (ICMS). Comparative statistical evaluation of the results from spike solutions/simulants, liquid radioactive waste samples, and interlaboratory performance test samples demonstrated that the various methods generated accurate and/or equivalent total mercury data and equivalent precision (2σ ± < 20%). Triplicate total mercury analysis of an exemplar radioactive waste resulted in an average value of 54.1 mg/L (as Hg) using CVAAS, 55.1 mg/L using ICPMS and 56.0 mg/L using DMA. A primary advantage of the DMA in a radioactive environment is avoiding multi-step, labor-intensive, time-consuming and waste-producing sample preparation protocols needed for CVAAS and ICPMS. DMA was determined to be the preferred method for measuring total mercury in the Savannah River National Laboratory (SRNL) radioanalytical laboratory based on analytical performance combined with ease of use in a radiological containment unit.
Article highlights
Direct mercury analysis (DMA) is well matched to the analytical constraints for radioactive samples.
DMA accuracy & precision were similar to other methods.
Deployment of DMA reduced analysis time, worker exposure, and radioactive waste generation.











Similar content being viewed by others
References
Committee on Separations Technology and Transmutation Systems (1996) Nuclear Wastes: Technologies for Separations and Transmutation. The National Academies Press, Washington, D.C.
Bannochie C, Fellinger T, Garcia-Strickland P, Shah H, Jain V, Wilmarth W (2017) Mercury in aqueous tank waste at the Savannah River Site: Facts, forms, and impacts. Sep Sci Technol 53:1935–1947
Mercury in Water by Manual Cold Vapor Atomic Absorption (CVAA) : EPA Method 245.1, United States Environmental Protection Agency, https://www.epa.gov/quality/mercury-water-manual-cold-vapor-atomic-absorption-cvaa-epa-method-2451, accessed 15 Jan 2020
Method 6020 A Inductively Coupled Plasma-Mass Spectrometry, United States Environmental Protection Agency, https://19january2017snapshot.epa.gov/sites/production/files/2015-07/documents/epa-6020a.pdf, accessed 15 Jan 2020
SW-846 Test Method 7473 : Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry, United States Environmental Protection Agency, https://www.epa.gov/hw-sw846/sw-846-test-method-7473-mercury-solids-and-solutions-thermal-decomposition-amalgamation-and, accessed 15 Jan 2020
Woodham W (2018) Run Plan for Testing to Evaluate Importance of Major Salt Species on Thermolytic Production of Hydrogen from Glycolate. SRNL-L3300-2018-00011 r 1. Savannah River National Laboratory, Aiken, SC
Looney B (2016) Scoping Studies for Advanced Oxidation Reactions for Transformation of Methylmercury in Alkaline Liquid Waste Simulants. SRNL-L3200-2016-00009. Savannah River National Laboratory, Aiken, SC
White TL, Brown LW, Looney BB, Jones MA(2019) Total Mercury Analysis Comparison: Deployment of Analytical Method for the Savannah River Site Liquid Waste System, SRNL-STI-2019-00056, Savannah River Site Aiken SC 29808
Phatak SE Your Direct Mercury Analysis: Sorbent Tube Gas Analysis,Milestone Inc, Shelton, CT
Shrivastava A, Gupta V (2011) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles of Young Scientists 2:21–25
Carrier O, Shahidzadeh-Bonn N, Zargar R, Aytouna M, Habibi M, Eggers J, Bonn D (2016) Evaporation of water: evaporation rate and collective effects. J Fluid Mech 798:774–786
Decadt G, Baeyens W, Bradley D, Goeyens L (1985) Determination of Methylmercury in Biological Samples by Semiautomated Headspace Analysis. Anal Chem 57:2788–2791
Slowey A (2010) Rate of formation and dissolution of mercury sulfide nanoparticles: The dual role of natural organic matter. Geochim et Cosmochim 74:4693–4708
Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry, United States Environmental Protection Agency, https://www.epa.gov/sites/production/files/2015-08/documents/method_1631e_2002.pdf, accessed 15 Jan 2020
Szakacs O, Lasztity A, Horvath Z (1980) Breakdown of Organic Mercury Compounds by Hydrochloric Acid-Permanganate or Bromine Monochloride solution for the Determination of Mercury by Cold Vapour Atomic Absorption Spectrometry. Anal Chim Acta 121:219–224
Fondeur F (2019) Solvent Hold Tank Sample Results for MCU-19-83-84-85: February 2019 Monthly Sample, SRNL-L3160-2019-00005. Savannah River National Laboratory, Aiken, SC
Acknowledgements
The authors wish to acknowledge the efforts of Sharon Gleaton and Lawrence Cheatham for preforming the analyses of samples on the DMA instrument. Additionally, we would like to thank Don Pak for modifying the DMA instrument for ease of use in a contamination area.
Funding
Savannah River National Laboratory is operated by Battelle Savannah River Alliance for the U.S. Department of Energy under Contract No. 89303321CEM000080. This work was supported by the US Department of Energy Environmental Management Office of Technology Development under Contract Number DE-AC09-08SR2 and the US Department of Energy National Nuclear Security Administration in collaboration with Savannah River Remediation under Contract No. DE-AC09-09SR22505. Publisher acknowledges the U.S. Government license to provide public access under the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Brian B. Looney, Leigh W. Brown, Thomas L. White and Mark A. Jones. The first draft of the manuscript was written by Thomas L. White and all authors contributed to editing and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could influence, or appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Looney, B.B., Brown, L.W., Jones, M.A. et al. Total Mercury Analysis of Radioactive Waste Containing Multiple Mercury Species. J Radioanal Nucl Chem 331, 4817–4828 (2022). https://doi.org/10.1007/s10967-022-08513-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08513-9