Abstract
The Chalmers Grouped ActiNide EXtraction process is a solvent extraction process for the homogeneous recycling of spent nuclear fuel. The use of TBP for the extraction of tetra- and hexavalent actinides can be problematic for several reasons, including troublesome degradation products causing crud formation, decreased extraction yield and the possibility of explosive red oil reactions. Here, the substitution of TBP by a N,N-dialkyl monoamide, DEHBA, is investigated. The findings suggest that DEHBA can be a suitable extracting agent for use in the CHALMEX solvent, although identified drawbacks need to be further investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
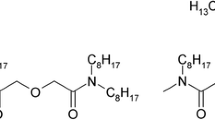
The recovery of tetra- and hexavalent actinides, predominantly uranium and plutonium, from irradiated nuclear fuel using the extractant tri-n-butyl phosphate (TBP) has been done on an industrial scale since the early 1950’s [1, 2]. TBPs high affinity and loading capacity for U(VI) and Pu(IV) made it the benchmark molecule for the separation of uranium and plutonium from fission products in the Plutonium Uranium Reduction Extraction (PUREX) process [1, 3, 4]. The molecules structure can be seen in Fig. 1 (left). TBP has a high resistance towards both radiolysis and hydrolysis. Its degradation products are primarily mono-butyl phosphate and dibutyl phosphate, and other less abundant degradation products. They are known to cause both red oil reactions and promote crud formation. Both aspects can have serious implications in reprocessing plants [5,6,7,8,9]. Furthermore, the presence of phosphor in the molecule is often referred to as being problematic in modern processes, in which the aim is typically to develop CHON-abiding processes. Adhering to the CHON-principle (only molecules containing C, H, O and N) allows for complete incineration of the final, spent solvent, thus minimizing the volume of the final waste [10]. However, the Chalmers GANEX (CHALMEX) diluent, phenyl trifluoromethyl sulfone (FS-13), which is seen in Fig. 1 (right), is both fluorinated and sulfonated. Here, solidifying the final organic waste has been suggested as a treatment option [11].
Possible alternatives to TBP as an extracting agent have received a lot of research efforts in the past decades. A promising group of extractants include the N,N-dialkyl monoamides, and especially the monoamide N,N-di-(2-ethylhexyl)butyramide (DEHBA), as seen in Fig. 2 [12,13,14,15,16]. Not only has the monoamide class of extractants been shown to be comparable or better than TBP in terms of U(VI) and Pu(IV) separation from the fission products, but they also have comparable radiolytic stability, compared to TBP [17,18,19,20,21,22,23]. Additional benefits of the N,N-dialkyl monoamide extractants are the less problematic degradation products. While TBP does not extract the lanthanides to any significant extent in the CHALMEX system, DEHBA yields D-ratios > 1 for both Sm and Eu in cyclohexanone [24]. Similar trends are seen for the corrosion products, where DEHBA consistently yielded higher D-ratios than TBP. Especially for Co and Mn, the D-ratios were > 1. For the fission product extraction however, DEHBA yields lower D- ratios than TBP for all the fission products with D > 1 (Mo, Pd, Ag, Cd and Sb), except for Zr [24].
The use of DEHiBA in the GANEX 1st cycle has been proven to recover more than 99.99% of the uranium, and so the main focus in this work is the ability of DEHBA to recover uranium and plutonium [25, 26].
Theory
The surface tension is a measure of the force required to form a surface on a liquid. Since liquid molecules at the surface are surrounded by fewer liquid molecules than the bulk liquid molecules, the surface molecules interact with each other to a higher extent than in the bulk liquid. Interfacial tension is practically the same force as surface tension, but between two liquids rather than between a liquid and air as for surface tension.
Both surface tension and density are important parameters to consider in solvent extraction as they play an important role in the formation and size of droplets. Generally, the smaller the droplet, the greater the surface area of contact between the two liquids and also the greater the mass transfer rate, at least up to a certain point. At one point the droplet size becomes so small that the droplets start behaving more like individual spheres and the rate of mass transfer will start to decrease again [27].
Density is an important parameter for the coalescence and separation of the organic and the aqueous phase. A sufficiently large density difference in a two-phase system will cause the two phases to spontaneously separate into two distinct layers. For systems with too similar densities between the phases, different phenomena can occur: phases can be “layered” vertically, i.e. side by side, or formation of a three-phase system can happen. In the latter, you’ll see parts of the heavy phase layered over the aqueous phase. It is also important to keep in mind that in solvent extraction processes in which the metal content is high, the density of the organic phase will increase as extraction proceeds, while it will decrease for the aqueous phase. In systems with a heavier aqueous phase and lighter organic phase, the density difference will thus decrease [28].
Surface active agents (surfactants) are molecules with a polar- and non-polar part, or hydrophobic and hydrophilic, respectively. In a solvent extraction system, a surfactant can be added to lower the surface tension. The surfactant will then dissolve its hydrophobic part in the organic phase, and its hydrophilic part in the aqueous phase. In systems in which the surfactant is also an extractant, an increase in surfactant/extractant concentration is typically associated with an increased rate of extraction [29].
Experimental
Unless otherwise stated, the DEHBA solvent constitutes 30% v/v DEHBA and 70% v/v FS-13, and the TBP solvent 30% v/v TBP and 70% v/v FS-13, pre-equilibrated with 4 M HNO3. The pre-equilibration is necessary to minimize effects of mutual solubility of the organic and aqueous phases in each other.
Physical properties of the solvents were measured using a tensiometer (Sigma 700, Attension, using a du Noüy ring). Each measurement was repeated at least twice, and the average measured value is reported here. Surface tension was measured against air, while interfacial tension (IFT) was measured between the solvent and 4 M HNO3. All measurements were performed at room temperature of 295 K.
The DEHBA solvent was irradiated using a 60Co gamma-source (Gamma cell 220, Atomic Energy of Canada ltd). To replicate process conditions, the solvent was irradiated aerated and in contact with 4 M HNO3. After irradiation, the organic phase was used for extractions from 4 M HNO3 immediately after collection.
For the acid extraction experiments, equal volumes (500 μL) of the respective organic phases were contacted with an equal volume of nitric acid with varying concentrations ranging from 0.01 to 4 M using an IKA Vibrax VXR, 1500 rpm shaker. The contact time was 15 min and the temperature controlled by a thermostatic bath (Grant Instruments, TC120 Heated Circulating Bath) at 298 K. Both initial acid concentration and the resulting acid concentration after contacting with the organic phase were titrated at least twice. The organic phase was then contacted with MQ water for 5 min, and the MQ water was titrated for mass balance purposes. Errors were taken as the mass balance deviation.
Batch solvent extraction was performed always using a phase ratio Θ = 1 and a minimum volume of each phase of 400 μL. Contacting was done using an IKA Vibrax VXR, 1500 rpm shaker and thermostatic bath. The radionuclides were added at trace concentrations and all radionuclides were investigated in isolated systems, except for Am and Eu which were investigated together. The activity of the radionuclides were 323 kBq mL−1 for Pu, 281 kBq mL−1 for Am, 278 kBq mL−1 for Eu and 30 kBq mL−1 for Np. The concentration of the U-stock solution was 1.1 M, diluted to 10−4 M using 4 M HNO3. All data points represent the average of triplicate samples, where the uncertainty is taken as the standard deviation of the series. Unless otherwise stated, the temperature was kept at 298 K. Save for the kinetics experiments, the contact time was 1 h for all the experiments which was enough to reach extraction equilibrium. After contacting, the samples were centrifuged for 5 min to ensure complete phase separation.
Analysis
Both 238Pu and 237Np were analysed by taking a 100 μL aliquot of each phase and measuring them using a liquid scintillation counter (LSC, Wallac 1414 WinSpectral). The samples were dissolved in 5 mL Ultima Gold. The aqueous phase of the natU-samples was diluted and measured using ICP-MS (Perkin Elmer NexION 2000C). 100 μL of each phase of the 241Am/152Eu system was measured on a high purity germanium detector (HPGe).
Results and discussion
The physical properties such as density, surface tension and interfacial tension plays a crucial role in solvent extraction. Here, the mentioned parameters have been measured for various versions of both the DEHBA- and the TBP-solvent, and the results are presented in Table 1.
DEHBA has a lower density than TBP, and when diluted in FS-13 the solvent density of the DEHBA solvent showed a lower density than the TBP solvent for the pre-equilibrated system. The density difference is significant with 1.28 g cm−3 for the TBP solvent and 1.12 g cm−3 for the DEHBA solvent, while the density difference is less pronounced for the pristine solvents with 1.26 g cm−3 for the TBP-solvent and 1.20 g cm−3 for the DEHBA solvent. The density of nitric acid is approximately 1.15 g cm−3 at 295 K and so the density difference to the pre-equilibrated DEHBA solvent is only 0.03 g cm−3 [30]. This can be a source of issues with phase separation under process conditions. Especially considering that metal extraction will cause a density increase of the solvent and a density decrease of the aqueous phase. This may in the worst case even lead to phase inversion, and so further hydrodynamic tests are necessary to determine the suitability of the DEHBA solvent for reprocessing applications.
The surface tension of the different solvents appears to be dominated by the extracting agent added. For both solvents, the surface tension is closer in value to that of the pure extractant, rather than to that of the pure diluent. The lower surface tension for FS-13 is somewhat surprising as it is not known to be very surface active despite its polar nature. The TBP solvent sees an increase in its surface tension upon pre-equilibration with 4 M HNO3, which can suggest a reorientation of the TBP molecule in the solvent. Any acid extracted by the ligand will not only occupy ligand, taking it away from the phase boundary, it can also change the charge density of the solvent. For the DEHBA solvent, the opposite trend is observed; an increase in the surface tension from the pristine solvent to the pre-equilibrated solvent. This could be due to the presence of water-soluble amine impurities, which is removed upon pre-equilibration.
Interfacial tension (IFT) is perhaps more interesting than the surface tension, as the former shows the force between the solvent and the nitric acid (4 M). In contrast to earlier work reported on DEHBA and TBP in cyclohexanone, the interfacial tension of the TBP solvent is higher than that of the DEHBA solvent, while for n-dodecane it is the other way around [16, 31]. It has previously been shown that DEHBA has a much larger IFT towards nitric acid compared to TBP, which indicates a much higher degree of surface activity for TBP [16]. Here, the higher interfacial tension of the TBP solvent can indicate that the interaction between TBP and FS-13 to some degree prevents the TBP molecule acting like a surfactant, through e.g. micelle formation. Micelle formation is a phenomenon in which the concentration of a surfactant becomes so high that they self-assemble in colloidal aggregates. The lower IFT for the DEHBA solvent also suggests a quicker mass transfer rate compared to the TBP solvent. A lower IFT produces smaller droplets and increased surface area of contact, which is known to promote faster mass transfer rates [27].
In earlier published work, it was found that DEHBA extracts acid to the same extent as TBP, although it was mainly attributed to extraction by the diluent cyclohexanone. In later work, acid extraction by TBP was investigated, and found to be relatively high, while no acid is extracted by the current FS-13 diluent [33]. Published results have also found that nitric acid extraction by TBP occurs by both a 1:2 and a 1:1 complex formation for HNO3:TBP [34, 35]. Here we show that the acid extraction by DEHBA is indeed comparable to the acid extraction by TBP, at least up until 2 M [HNO3], as shown in Fig. 3. A slope analysis of the log(HNO3) vs log(D(DEHBA)) plot yields no conclusive dependency with a slope of 0.73 and R2 = 0.85. This indicates mixed complexes of HNO3-DEHBA. At [HNO3] > 2 M, extraction is still significant, but the extraction by TBP exceeds that of DEHBA. For both solvents, the acid extraction is a function of the acid concentration, although it appears that the acid extraction of TBP reaches its maximum at 4 M HNO3. Therefore, with the use of the DEHBA solvent, an additional acid scrubbing step would need to be included within the process.
[H+] extracted by the organic phase versus initial acid concentration. Data points for TBP solvent at 1, 4 and 5 M HNO3 are reproduced from [33]
Extraction tests were performed for all the actinides of interest, irrespective of expected oxidation state in the spent nuclear fuel raffinate. The results at equilibrium are presented in Table 2. None of the trivalent or pentavalent actinides are extracted to any significant degree, with D-ratios < 0.01 for Np(V), Am(III) and Eu(III). This was expected as DEHBA is known to extract the tetra- and hexavalent actinides. It has previously been found that Np(VI) is extractable with D > 1 by DEHBA from nitric acid solutions with nitrate concentration above 1 M, while D-ratios of Np(V) remained < 1 for the nitric acid range investigated [36]. While the oxidation state of Np(V) was confirmed by UV–VIS for these experiments, the speciation of Np has been shown to spontaneously distribute between Np(V, VI). Furthermore, Np is sensitive to both oxidizing and reducing agents (i.e., nitrous acid), and temperature- and acid concentration changes. The oxidation state of Np was challenging to control under the given process conditions [37,38,39,40,41,42]. In conclusion, it is expected that the Np oxidation state will be a mixture of + 5 and + 6 in spent nuclear fuel raffinate.
The distribution ratios, at equilibrium, for Pu(IV) and U(VI), were 11 and 9.4, respectively. These D-ratios yield a high separation factor over the lanthanides, here represented by Eu(III). Compared to TBP, the D-ratios are once again comparable. Halleröd et al. [43] reported D(Pu) of approximately 12 and D(U) of approximately 10.5, while D(Am) and D(Eu) were < 0.1. The D-ratios of Np cannot be directly compared as Np(V,VI) was used for the extraction by TBP in FS-13.
Pu has been shown to form both a 2:1 and a 1:1 complex with TBP. In the CHALMEX FS-13 system, the slope of Pu extraction as a function of TBP concentration is 1.27, which could be a product of co-extraction by CyMe4-BTBP or adduct formation with nitric acid [33]. For the DEHBA solvent, the slope of Pu extraction shows a 2:1 complex formation, as seen in Fig. 4. This agrees with results published earlier [44]. In addition, this publication provided evidence for a 3:1 complex, which is not seen for the CHALMEX system. For uranium, a slope of 1 is seen which suggests a 1:1 complex formation with the DEHBA ligand, while a thorough study by Acher et al. showed that uranium is coordinated by two DEHBA molecules and 4 nitrate ions [44]. Slope analysis is a less robust method compared to spectroscopic evidence such as those used by Acher et al. Furthermore, the use of different diluents can affect the complexation of ligand to metal.
The nitric acid dependency was also investigated, and the results are presented in Fig. 5. As can be seen, the D-ratios increase with increasing nitric acid for both U and Pu. The slope of D(Pu) is steeper (0.994) compared to the slope of D(U) (0.535), suggesting a stronger dependency on the nitrate content of the aqueous solution. The same tests were performed by changing the nitrate concentration only (using NaNO3), which yielded the same slope for Pu. This confirms that the extraction is dependent on the nitrate concentration, rather than the acid concentration.
Earlier studies have shown that the presence of TBP in the solvent more readily promotes the hydrolytic degradation of the BTBP-molecule [17]. For comparison, no BTBP degradation products were detected in the DEHBA solvent for the same conditions and exposure time. Although not relevant for the extraction of the tetra- and hexavalent actinides, the degradation of CyMe4-BTBP led to a significant decrease in both Am and Eu D-ratios. A decrease of Am and Eu D-ratios were seen also for the DEHBA solvent in the presence of CyMe4-BTBP, which was attributed to water soluble DEHBA degradation products acting like masking agents for Am and Eu. For both solvents, a more problematic decrease in Np D-ratios was seen as hydrolysis progressed, mainly due to the low original D(Np) [17].
Similar, though accelerated, trends were seen for the radiolytic stability of the DEHBA solvent, whether it be linked to the loss of extracting agent or the presence of water soluble “complexing agents” (DEHBA degradation products), as shown in Table 3. For Pu, a higher D-ratio than the unirradiated value (Deqm = 11.4) is observed for all doses. The highest D-ratio is seen after only 5 kGy, with D = 27.2, with consistently decreasing D-ratios with increasing doses. For the distribution ratio of U, the observations are less consistent. At 5 kGy, D = 13.8, which is higher than its equilibrium value (Deqm = 9.41). This is also observed for a dose of 100 kGy, where D = 9.51 compared to D = 8.12 for 75 kGy. This could be due to the formation of less stable degradation products, which have a higher affinity for U than the original DEHBA molecule.
For the extraction of uranium and plutonium as a function of time, both ligands reach extraction equilibrium within five minutes of contact time. A surprising trend is seen in Fig. 6 for the extraction of U and Pu by DEHBA. The standard deviations of the triplicates were generally below 5% for all dose rates, except for D(Pu) at t = 1 min, for which the uncertainty was 13% and D(U) at t = 3 min and t = 10, for which the uncertainties were 11% and 25% respectively. The uncertainties make little to no difference in the trends seen for either Pu or U. The highest D-ratios are seen after 3 min of contacting, before the D-ratios drop to values close to their equilibrium values. This is quite unexpected behavior, but it could be partially due to difficulties in ensuring accurate contact times. Another possibility is that unknown and less controlled chemical reactions are happening during the first minutes of contacting. If so, it is probably a reaction between the metal and the solvent, as the solvent is already pre-equilibrated with 4 M nitric acid. Further investigations are required to understand what is causing this phenomenon. Overall, the distribution ratios are high for the DEHBA solvent and can be compared to those of the TBP solvent published by Halleröd et al. [43].
Conclusions
In this study, a comparison of DEHBA and TBP was made for the extraction of the tetravalent and hexavalent actinides, namely U(VI) and Pu(IV). Several performance criteria were investigated, including the physical properties of the solvents, acid extraction by the ligands, distribution ratios at extraction equilibrium, extraction kinetics, extraction as a function of ligand concentration and nitric acid concentration, and radiolytic stability. While the ligands are comparable for most of the evaluation criteria investigated here, some differences were identified. The acid extraction by the TBP solvent is higher than that of the DEHBA solvent at [HNO3] > 2 M. Some concerns were identified in the current work for the DEHBA solvent; its low density and the low interfacial tension between the solvent and 4 M nitric acid. Both these properties can cause serious phase inversion issues and/or phase separation issues under process conditions. It is thus suggested that future work focuses on investigations of the hydrodynamics of the DEHBA solvent to determine its suitability for use in the CHALMEX process. Subsequently, further studies can also include investigations of co-extraction of fission products in the FS-13 diluent as well as investigations of the radiolytic degradation products.
References
Irish ER, Reas WH (1957) The PUREX PROCESS— a solvent extraction reprocessing method for irradiated uranium. General Electric, Richland, Washington
Thompson SG, Seaborg GT (1944) Bismuth phosphate process for the separation of plutonium from aqueous solutions, US Patent Office (USPTO) USA
PUREX process, European Nuclear Society (2017) https://www.euronuclear.org/glossary/purex-process/
Richardson GL (1964) The design and operation of purex process pulse columns in chemical engineering development. Hanford Laboratories Operation, Richland, Washington
Wright A, Paviet-Hartmann P (2010) Review of physical and chemical properties of tributyl phosphate/diluent/nitric acid systems. Sep Sci Technol 45(12–13):1753–1762
Usachev VN, Markov GS (2003) Incidents caused by red oil phenomena at semi-scale and industrial radiochemical units. Radiochemistry 45(1):1–8
Higgins CE, Baldwin WH (1961) The thermal decomposition of tributyl phosphate. J Org Chem 26(3):846–850
Paddleford D, Fauske H (1994) Safe venting of red oil runaway reactions. Westinghouse Savannah River Co., Savannah River, South Carolina
Stieglitz L, Becker R (1985) Chemical and radiolytical solvent degradation in the Purex process. Atomkernenerg Kerntech 46(2):76–80
Madic C, Hudson MJ (1998) High-level liquid waste partitioning by means of completely incinerable extractants. European Commission, DG RTD, Brussels, Belgium
Rzhekhina EK, Karkozov VG, Alyapyshev M, Baibain VA (2007) Reprocessing of spent solvent of the UNEX process. Radiochemistry 49(5):493–498
Horne GP, Zarzana CA, Rae C, Schmitt NC, Duane Ball R, Tillotson RD, Mezyk SP, Mincher BJ, Ceder K, Charbonnel MC, Berthon L, Guilbaud P, Saint-Louis G (2020) DEHBA (di-2-ethylhexylbutyramide) gamma radiolysis under spent nuclear fuel solvent extraction process conditions. Radiat Phys Chem 170(5):108608
Pathak PN, Kumbhare LB, Manchanda VK (2001) Effect of structure of N, N dialkyl amides on the extraction of U(VI) and Th(IV): a thermodynamic study. Radiochim Acta 89(7):447–452
Pathak PN, Veeraraghavan R, Pranhu DR, Mahajan GR, Manchanda VK (1999) Separation studies of uranium and thorium using Di-2-ethylhexyl isobutyramide (D2EHIBA). Sep Sci Technol 34(13):2601–2614
Benay G, Wipff G (2013) Liquid-Liquid extraction of uranyl by an amide ligand: interfacial features studied by MD and PMF simulations. J Phys Chem 117(24):7399–7415
Pathak PN, Kanekar AS, Prabhu D, Manchanda V (2009) Comparison of hydrometallurgical parameters of N, N-dialkylamides and of Tri-n-butylphosphate. Solvent Extr Ion Exch 27(5–6):683–694
Aneheim E, Ekberg C, Foreman MRS, Löfström-Engdahl E, Mabile N (2012) Studies of a solvent for GANEX applications containing CyMe4-BTBP and DEHBA in cyclohexanone. Sep Sci Technol 47(5):663–669
Nair GM, Mahajan GR, Prabhu DR (1995) Extraction of uranium(VI) and plutonium(IV) with some high molecular weight aliphatic monoamides from nitric acid medium. J Radioanal Nucl Chem 191(2):23–330
Prabhu DR, Mahajan GR, Nair GM (1997) Di(2-ethyl hexyl) butyramide and di(2-ethyl hexyl)isobutyramide as extractants for uranium(VI) and plutonium(IV). J Radioanal Nucl Chem 224(1):113–117
Pathak P (2014) N, N-Dialkyl amides as extractants for spent fuel reprocessing: an overview. J Radioanal Nucl Chem 300(1):7–15
Gasparini GM, Grossi G (1986) Review article long chain disubstituted aliphatic amides as extracting agents in industrial applications of solvent extraction. Solvent Extr Ion Exch 4(6):1233–1271
Suzuki S, Sasaki Y, Yaita T, Kimura T (2004) Study on selective separation of uranium by N,N-dialkyl-amide in ARTIST process. Japan Atomic Energy Research Institute, Tokai-mura, Japan
Verma PK, Pathak PN, Kumari N, Sadhu B, Sundararajan M, Aswal VK, Mohapatra PK (2014) Effect of successive alkylation of N, N-dialkyl amides on the complexation behavior of uranium and thorium: solvent extraction, small angle neutron scattering, and computational studies. J Phys Chem 118(49):14388–14396
Aneheim E, Ekberg C, Mabile N (2011) Exchange of TBP for a monoamide extraction ligand in a GANEX solvent- advantages and disadvantages. In: Proceedings in ISEC. Santiago, Chile
Miguirditchian M, Sorel C, Cames B, Bisel I, Baron P (2008) Extraction of uranium(VI) by N,N-di-(2-ethylhexyl)isobutyramide (DEHIBA): from the batch experimental data to the countercurrent process. In: Proceedings in ISEC. Tucson, Arizona
Miguirditchian M, Sorel C, Cames B, Bisel I, Baron P, Espinoux D, Calor JN, Viallesoubranne C, Lorrain B, Masson M (2009) HA demonstration in the atalante facility of the GANEX 1st cycle for the selective extraction of Uranium from HLW. In: Proceedings in GLOBAL. Paris, France
Rydberg J, Cox M, Musikas C, Choppin GR (2004) Solvent extraction principles and practice, 2nd edn. Marcel Dekker, New York, New York
Löfström-Engdahl E (2014) On the diluent and solvent effects in liquid-liquid extraction systems based on Bis-Triazine-Bipyridine ligands. Chalmers University of Technology, Gothenburg, Sweden
Chaiko D, Osseo-Asare K (1990) Monolayer behavior of surface active metal extractants in surfactants in solution. Springer, Boston, Massachusetts
The Complete Aqueous Nitric Acid Solutions Density-Concentration Calculator, Handymath (2022) https://www.handymath.com/cgi-bin/nitrictble2.cgi?submit=Entry
Löfström-Engdahl E, Aneheim E, Ekberg C, Foreman M, Skarnemark G (2013) Comparison of the extraction as a function of time in two GANEX solvents: influence of metal loading, interfacial tension, and density. Solvent Extr Ion Exch 31(6):604–616
Halleröd J, Ekberg C, Kajan I, Aneheim E (2018) Solubility thermodynamics of CyMe4 -BTBP in various diluents mixed with TBP. J Solution Chem 47(6):1021–1036
Authen TL, Wilden A, Halleröd J, Schneider D, Kreft F, Modolo G, Ekberg C (2020) Batch tests for optimisation of solvent composition and process flexibility of the CHALMEX FS-13 process. Solvent Extr Ion Exch 39(1):1–17
Chaiko DJ, Vandegrift GF (1988) A thermodynamic model of nitric acid extraction by Tri-n-Butyl phosphate. Nucl Technol 82(1):52–59
Alcock K, Grimley SS, Healey TV, Kennedy J, McKay HAC (1956) The extraction of nitrates by tri-n-butyl phosphate (TBP). Part 1.—The system TBP + Diluent + H2O+HNO3. J Chem Soc Faraday Trans 52:39–47
Ban Y, Hotoku S, Tsutsui N, Tsubata Y, Matsumura T (2016) Distribution behavior of neptunium by extraction with N, N-dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractors. Solvent Extr Ion Exch 34(1):37–47
Precek M, Paulenova A, Mincher BJ (2012) Reduction of Np(VI) in irradiated solutions of nitric acid. Procedia Chem 7:51–58
Gregson C, Boxall C, Carrott M, Edwards S, Sarsfield M, Taylor R, Woodhead D (2012) Neptunium (V) oxidation by nitrous acid in nitric acid. Procedia Chem 7:398–403
Chatterjee S, Bryan SA, Casella AJ, Peterson JM, Levitskaia TG (2017) Mechanisms of neptunium redox reactions in nitric acid solutions. Inorg Chem Front 4(4):581–594
Marchenko VI, Koltunov VS, Dvoeglazov KN (2010) Kinetics and mechanisms of redox reactions of U, Pu, and Np in tributyl phosphate solutions. Radiochemistry 52(2):111–126
Zhang H, Liu Z, Zhou X, Li L (2017) The complex reaction kinetics of neptunium including redox and extraction process in 30% TBP—nitric acid system. J Radioanal Nucl Chem 312(2):173–180
Shilov VP, Gogolev AV, Fedoseev AM (2012) Behavior of neptunium ions in organic media. Radiochemistry 54(4):315–323
Halleröd J, Ekberg C, Authen T, Bertolo L, Lin M, Grüner B, Švehla J, Wagner C, Geist A, Panak P, Aneheim E (2018) On the basic extraction properties of a phenyl trifluoromethyl sulfone-based GANEX system containing CyMe4-BTBP and TBP. Solvent Extr Ion Exch 36(4):360–372
Acher E, Cherkaski YH, Dumas T, Tamain C, Guillaumont D, Boubals N, Javierre G, Hennig C, Solari PL, Charbonnel MC (2016) Structures of plutonium (IV) and uranium (VI) with N, N-dialkyl amides from crystallography, X-ray absorption spectra, and theoretical calculations. Inorg Chem 55(11):5558–5569
Acknowledgements
H2020 Euratom Research and Innovation Programme under Grant agreement no 755171 is acknowledged for funding this research.
Funding
Open access funding provided by Chalmers University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Authen, T.L., Tekikachew, B.E., Foreman, M.R.S.J. et al. A comparison on the use of DEHBA or TBP as extracting agent for tetra- and hexavalent actinides in the CHALMEX Process. J Radioanal Nucl Chem 331, 5137–5145 (2022). https://doi.org/10.1007/s10967-022-08481-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08481-0