Abstract
Pretreated silica sand labeling using varying concentrations of tin(II) fluoride and chloride as reducing agents and different times labeling was performed in order to develop a methodology for labeling silica sand with 99mTc for using as solid radiotracer. Influence of different sand pretreatment parameters on the sorption yield (Rret%) was statistically evaluated. The effectiveness of the methods used to reduce pertechnetate (99mTcO4−) by ascending paper chromatography was confirmed. Results show relatively high values of 99mTc sorption yields on silica sand. It was possible to establish a methodology for obtaining solid 99mTc labeled radiotracers in support of silica sand.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Radiotracers as a tool for various applications in the industry allow to carry out studies without stopping the technological flow of processes. However, this technique is still underutilized. The main cause is the lack of timely availability of required radiotracer [1]. Studies which expand the potentialities of 99mTc eluted from 99Mo/99mTc generator to label silts [2], silica (FS) and zeolite (FZ) supported ferragels, surface waters in non-reducing [3, 4], or reducing environment [5], and to trace the organic liquid phase [6], have been carried out in our country. Silica sand and others compounds with a high SiO2 content are frequently used in industrial applications [7]. On the other hand, processes involving materials of similar density, granulometry and specific weight to the silica sand could also be studied with labeled silica sand [7]. The labeling of silica sand with 99mTc is shown as an attractive option to ensure the availability of radiotracers required in industrial applications.
Labeling of sands with radioisotopes has been approached previously [8, 9] using methods of activation of lanthanum oxides. Techniques of this type are not available in countries that don’t have nuclear facilities for the activation of solid matrixes. The reduction method of 99mTcO4− with tin(II) chloride (SnCl2) for labeling of silica sand has been used by others authors [10]. These studies are still in a preliminary level. The labeling methodology is not very described in the literature neither sufficiently studied.
On the other hand, several authors in the Radiopharmacy field have used tin(II) fluoride (SnF2) instead of the SnCl2 to reduce the 99mTc, adducing its smallest tendency to the hydrate and therefore its biggest storage stability [11,12,13].
In the present work, the pretreated silica sand was labeled by 99mTcO4− reduction using different times labeling and varying concentrations of SnCl2 and SnF2 as reducing agents in order to get a solid 99mTc radiotracer for multiphase flow studies. The purpose of this work is to evaluate the influence of different parameters on the pretreatment and labeling silica sand in order to obtain a prospective 99mTc solid radiotracer.
Experimental
1 g of silica sand and 1 mL of 99mTcO4− (ac) eluted from the 99Mo/99mTc generator with 3 MBq of 99mTc activity were added in a tube for centrifuge. The mixture was stirred for a period of 15 min. Soon afterwards 2 mL of SnF2 or SnCl2 solution (pH = 5) of varying concentration according to experiment, was added. The solution was stirred for a reaction time which varies in each experiment and it was centrifuged for 1 min. Three aqueous phase samples of 0.2 mL were taken and were measured three times with a radiometric system SRN1C-02, coupled to a NaI (Tl) detector. To evaluate 99mTc retention in the silica sand, the retention degree (Rret%), was determined indirectly as:
where, Al (cps mL–1) and Ab (cps mL–1) are the radioactive concentrations of 99mTc solutions, after and before the contact with the silica sand, respectively. Each experiment was repeated three times for error estimation. Some studies were carried out using experimental designs. In these cases, the error was calculated from three experiments in the center of the experimental plan. Cuban natural silica sand from Silica Sand Industrial Plant of Guane, Pinar del Río, was used.
Pretreatment of silica sand was conducted in two main stages. In the first, 15 g of silica sand was put in contact with 15 mL of concentrated nitric acid (HNO3) and the mixture was stirred for 15 min (tHNO3−sand) in a magnetic stirrer. Vacuum filtration with 25 mL of distilled water was used to separate the silica sand from the liquid phase. After filtered sand spread in a peatry plate and allowed to dry with IR lamp for 30 min. For the second stage the solid was transferred to a beaker in which were added 15 mL of sodium hydroxide (NaOH) 2 mol L− 1 of concentration and kept in touch with constant stirring for 15 min (tNaOH−sand), filtered under vacuum filtration and the solid was dried with IR lamp for 1 h.
Paper chromatography with acetone as mobile phase was employed to verify the percent of reduced 99mTcO4− to 99mTc colloidal - hydrolyzated (99mTcO2, 99mTcO(OH)2 or 99mTc2+) with SnCl2 or SnF2. Afterwards 2 mL of SnCl2 or SnF2 solution of varying concentration were added (according to experiment) to 1 mL of 99mTcO4− (ac) and the solution was stirred for the proposed reaction time for each experiment. A drop is removed and placed at the origin of the paper strip, this was placed in the tank to chromatography (glass jars) containing acetone (2 mm deep) with the end of the origin downwards. When the front of the run was moved by the paper strip, this is removed and allowed to dry. The center strip was cut and measured in SRN1C-02 system, coupled to a NaI (Tl) detector [5, 6]. To evaluate the reduced 99mTc retention by chromatography, the reduction yield (R*%) was determined as:
,
where Aa (cps mL− 1) is the radioactive concentration at the point of application and Af (cps mL− 1) is the radioactive concentration in the solvent front. Yields are reported with standard deviations reflect three replicates, and three measurements per replicate for a total of 9 radiometric measurements for each value of R*%.
The type of reducing agent (SnCl2 or SnF2) influence on 99mTc retention in the silica sand (Rret%) was evaluated. Two experiments were carried out using 15 min of reaction time and 0.5 mmol L− 1 of concentration for each reducing agent. These conditions were chosen from previous studies results [10, 14].
A 22 factorial experimental plan was designed to study the influence of SnF2 concentration and reaction time on 99mTc retention in the solid. The maximum and minimum values of the plan used were 30 min and 15 min of reaction times, and 2 mmol L− 1 and 0.5 mmol L− 1 of SnF2 concentrations, respectively. Parameters values were selected taking into account the divergence from bibliographic reports and previous results [10, 15].
Some experiments to evaluate the influence on the 99mTc retention degree of the c(NaOH), relation V(HNO3)/m(sand), reaction time between HNO3 and sand (tHNO3−sand) and reaction time between NaOH and sand (tNaOH−sand) used for the pretreatment of the silica sand, were executed. A 24 factorial experimental plan was designed. Limits and the middle values of the plan used for each parameter are shown in Table 1. Parameters values were selected taking into account the divergence from bibliographic reports and previous results [10].
The data was processed with STATGRAPHICS Centurion XV software. P values for 5% degree of confidence were selected to determine statistical significance of the principal effects as well as the interactions. Three replicates were performed in the center of the plane to assess the pure error
In order to meet the transformations in silica sand after treatment a study of X-ray spectroscopy coupled to the technique of scanning electron microscopy (SEM-EDS) to samples of silica sand was made before and after treatment. For treatment of it there were employed c(NaOH) = 1 mol L− 1, V (HNO3)/m(sand) = 1.5, tHNO3−sand = 20 min and tNaOH−sand = 20 min
Results and discussion
With the purpose of relating the quantity of reduced 99mTcO4− (R*%) with the obtained retention degree (Rret%) the chromatographic study of the reduction of the 99mTcO4− was carried out. The results show that the reduction yields (R*%), with both reducing agents, were much higher than retention degrees obtained (Table 2), therefore there is a greater amount of reduced 99mTc species adsorbed on the surface of silica sand [12, 16].
Table 2 shows that obtained values (Rret%) using SnF2 are higher by approximately 20% than those obtained under the same conditions using SnCl2, probably due to the greater stability of SnF2 reducing properties [17].
There are different approaches in the literature about the influence of SnF2 concentration and reaction time on 99mTc retention in the silica sand [10]. The Rret % obtained from the 22 executed experimental design and P values for the principal effects as well as for their interactions are shown in Table 3. From the P value analysis, it was clear that just the variation of reaction time (B) between SnF2 and 99mTcO4− is statistically significant (P = 0.002). There is only a 0.2% probability that variation observed in the Rret% due to variation of parameter B could be by chance. The remaining factors (A and AB) are not statistically significant for a 95% confidence.
The highest 99mTc sorption yields were obtained for experiments where corresponding reaction times were employed at the minimum level (15 min), indicating that the increase of this factor has a negative effect on the degree of 99mTc retention.
Equation 3 describes the dependency between the Rret% and the studied parameters. Lack of fitting P value (0.6503) greater than 0.05 indicates that the model properly represents the behavior of the Rret% for a 95% of confidence in the studied interval.
Response surface of the Rret% model calculated is shown in Fig. 1. This allows to predict the Rret% with the variation of the factors within limits established in the design.
The results of chromatographic study in order to explore the cause of the negative effect of reaction time on the degree of 99mTc retention (Table 4) show a decrease in the values of R*% with increasing reaction time for both concentrations of reducing agent. This is probably the main cause of the decrease in the degree of retention of 99mTc in the treated silica sand with increasing to 30 min of reaction time.
Table 5 shows the results from 24 experimental design. From the P value analysis, it was clear that only factors B (V(HNO3)/m(sand)) and C (tHNO3−sand), within established limits, had significant effects on the degree of 99mTc retention. So, the observed variations in the Rret% are mainly provoked for the variation of the relation V(HNO3)/m(sand) and the reaction time between HNO3 and sand.
Indeed, higher Rret% values were obtained in experiments 1, 10, 17 and 19, which matched the maximum of V(HNO3)/m(sand) and tHNO3−sand, and the experiment 2, which corresponds to maximum value of tHNO3−sand, all obtained values are above 70%.
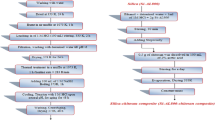
Figure 2 shows the semi-quantitative analysis of the composition of the silica sand sample before and after treatment, obtained by X-ray spectroscopy coupled to the technique Microscopy Scanning Electron Microscopy (SEM-EDS).
These results show the effect of each stage of the treatment, the first with HNO3 to clean the sand of organic matter, which is reflected in the study with the variation of the contents of oxygen and carbon. With the removal of these impurities are released pores on the surface of the grains that were previously occupied by them. During the second stage (treatment with NaOH) can modify the crystalline structure of SiO2, thereby increasing the available sites for adsorption. This is reflected from the silicon content decreased as from forming a gel of soluble silicate, this contact is washed with water and no longer part of the crystal structure [18]. The results discussed above suggest that the Rret% increased after treatment, are mainly due to the observed changes in silica sand chemical composition and structure.
Conclusions
The retention degree of 99mTc increase from 48.7 ± 1.4% when SnCl2 is used as reducing agent to 69.1 ± 0.1% with SnF2. So, the obtained results indicate that SnF2 is prospective for the labeling procedure. It can be stated with 95% confidence that in the range studied, the reaction time between SnF2 and 99mTcO4– influences negatively on the labeling of treated silica sand, while the concentration of SnF2 does not influence. It is recommended to use 15 min as reaction time and c(SnF2) = 0.5 mmol L–1 for labeling. The SEM-EDS analysis showed that the pretreatment of silica sand favors the 99mTc retention, probably due to the changes observed in its chemical composition and structure. Using 20 min for both reaction times (tHNO3-sand and tNaOH- sand), relation V(HNO3)/m(sand) = 1.5 and c(NaOH) = 1 mol L–1 for silica sand treatment a 99mTc retention degree of 74.5 ± 0.5% could be obtained. From the obtained results for treatment and labeling of Cuban silica sand, it was possible to establish an optimum methodology to obtain a solid 99mTc radiotracer for industry purposes.
References
IAEA (2004) Radiotracer applications in industry – a guidebook, in Technical Report, S.n. 423, Editor. International Atomic Energy Agency: Vienna
Gómez HR, Rey S, Cuello O (1973) Determinación del transporte de materia en un horno rotatorio de cemento, utilizando radioisótopos. CNEA, Argentina
Domínguez J, Borroto J, Hernández A (2003) Empleo de trazadores en la obtención de modelos de calidad de agua del río Almendares. Nucleus 34:19
Ito K, Akiba K (1991) Adsorption of pertechnetate ion on active carbon from acids and their salt solutions. J Radioanal Nucl Chem 152(2):381–390
Liang L, Gu B, Yin X (1996) Removal of technetium-99 from contaminated groundwater with sorbents and reductive materials. Sep Technol 6(2):111–122
Liu D, Fan X (2005) Adsorption behavior of 99Tc on Fe, Fe2O3 and Fe2O4. J Radioanal Nucl Chem 264(3):691–698
Pincay L, Amen H, Lung J (2009) Uso de sílice en hormigones de alto desempeño. Facultad de Ingeniería en Ciencias de la Tierra. Escuela Superior Politécnica del Litoral, Guayaquil, Ecuador
Kolar ZI(2005) y col. Preparation of 56Mn-labelled sand: A radiotracer for process industry oriented powder mixing studies. J Radioanal Nucl Chem 146(6): 391–400
Lachica y Baró (1963) Estudio del movimiento de arenas en las cercanías del Puerto de Mar del Plata usando arena marcada con 110Ag. Informe No. 100, CONF-188-2. República Argentina, Comisión Nacional de Energía Atómica
IAEA (2013) Radiotracer generators for industrial applications. Radiation Technology S. N. XXX. Vienna, International Atomic Energy Agency
Hernández A(2005) y col. Marcaje de ciprofloxacina con 99mTc para el diagnóstico de infección activa. Ciencias Biológicas. R. Cenic. Cuba, Centro Nacional de Investigaciones Científicas. N°36
Navarro G(2011) y col. Marcado con 99mTc de liposomas convencionales y su evaluación biológica. A. Journal. Cátedra de Farmacotecnia, Dpto. Cienfar, Universidad de la República, Montevideo, Uruguay. N°51
Srivastava SC(1977) y col. Problems Associated in Stannouese 99mTc- radiopharmaceutic. International Journal of Applied Radiation and Isotopes. New York, U.S.A, Department of Applied Science. 28: 83–95
Borroto J(2003) Comportamiento del 99mTc como radiotrazador en aguas superficiales y residuales. Tesis en opción al título de Doctor en Ciencias, Instituto Superior de Tecnologías y Ciencias Aplicadas: La Habana pp 16–38
Borroto J, Domínguez J(2003) Technetium-99m as a tracer for the liquid RTD measurement in opaque anaerobic digester: application in a sugar wastewater treatment plant., in Chemical Engineering and Processing p. 857–865
Prats A et al (2005) Ácido 3-amino-1-hidroxipropano-1,1-bisfosfónico en oncología: Síntesis y biodistribución, in Ciencias Químicas, R. CENIC, Editor. Centro Nacional de Investigaciones Científicas, Cuba
Hernández A (2009) Marcaje de ciprofloxacina con 99mTc para el diagnóstico de infecciones. Comparación y evaluación preclínica de dos métodos. Centro de Investigaciones Clínicas (ClC). Centro Nacional de Investigaciones Científicas (CNIC), La Habana, Cuba
Segarra J (2005) Envejecimiento de presas por reacciones expansivas en el hormigón, Las Reacciones Expansivas. Editor Nuevo Milenio, Cuba
Acknowledgements
We wish to thank the IAEA for supporting this work under the Coordinated Research Project (CRP) Radiometric Methods for Measuring and Modeling Multiphase Systems towards Industrial Processes.
Funding
Open access funding provided by University of Geneva
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montesino, L.E.L., Corrales, Y.A., Páez, A.D. et al. Treatment and labeling of silica sand for obtaining a prospective solid 99mTc radiotracer. J Radioanal Nucl Chem 331, 3359–3364 (2022). https://doi.org/10.1007/s10967-022-08424-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08424-9