Abstract
This study aimed to synthesize a new pyrimidine derivative with a good synthesis yield of 87% to act as a new cancer marker after radiolabeling with Tc-99m in a high radiochemical yield of 92.3%. In-vivo study in tumor-bearing Swiss albino mice model revealed promising data with high uptake in cancer. Docking study showed good binding interactions of the radiosynthesized complex at the binding site. In conclusion, this novel complex could be a potential probe for cancer targeting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer remains one of the leading causes of death worldwide, which makes the identification of novel drugs crucial to address this disease [1,2,3]. Moreover, cyclin-dependent kinases (CDKs) are a group of serine/threonine kinases comprising 20 members, of which some are linked with the regulation of cell-cycle progression by phosphorylating proteins involved in cell division [4, 5], and they are considered important targets for anticancer drugs [7, 8]. Heterocyclic and fused heterocyclic compounds were evaluated as potential anticancer agents. Pyrimidine is one of the main key groups in many antitumor, antitubercular, anti-inflammatory, antimicrobial, anti-HIV, antimalarial, analgesic drugs and cardiovascular agents, so a lot of scientists focusing on designing new compounds having a pyrimidine moiety [6]. The pyrimidine heterocyclic derivatives are of immense significance in their biological in addition to their synthetic reveal of medicinal chemistry essentially subsequent to the finding of Fluorouracil (1) [7], Tegafur (2) [8, 9], Uramustine (3) [9], Thiarabine (4) [10], Capecitabine (5) [11], Trimetrexate (6) Fig. 1 [12]. Furthermore, surveying literature revealed that N-alkylation of aminopyrimidine through the incorporation of the amino side chain in pyrimidine core has been reported to enhance the antitumor activity of candidate compounds. For example, ZK-304709 (7), an oral multi-target tumor growth inhibitor was reported to block tumor cell proliferation and induce apoptosis [13, 14]. Recently, through optimization of the linker-length of alkylamine in pyrimidine ring, Chen et al. discovered compound 8 which has potent activities inhibiting growth and migration of breast cancer cells, indicating the flexibility of the alkylamine moiety may have a critical importance for antiproliferative activity [15]. The aforementioned facts encouraged us to synthesize the target hybrid (9) bearing pyrimidine and alkylamine scaffolds (Fig. 1). Both medical science advancement and early disease detection are crucial tools for successful treatment approaches [16,17,18,19]. Nuclear medicine offers good imaging tools (PET and SPECT) that support detailed pictures of tumors [18, 20], and poses less risk to patients compared to surgical visualization [21, 22]. 99mTc is the most widely used radionuclide due to its favorable decay characteristics (t1/2 = 6.02 h, γ-ray emission = 140 keV, low cost and good availability) [23,24,25,26]. Nitrogen containing compounds are very strong chelators for technetium-99m, many complexes which having ligands containing amine nitrogen atoms have been reported as chelators for technetium-99m via one or more nitrogen atoms such as: [99mTc]Tc-2-nitroimidazole cyclam derivatives [27], [99mTc]Tc(CO)3· (IDA–PEG3–CB)]2 [28], [99mTc]Tc-HL91 [29], 2-, 4-, 5-substituted nitroimidazole–iminodiacetic acid–[99mTc]Tc(CO)3 complexes [30], [99mTc]Tc(CO)3-labeled chlorambucil analog [31] [99mTc]Tc-nitroimidazole complexes [32] and [99mTc]Tc-PyDA [33].
Experimental
All chemicals were purchased from Merck Company (Germany) with high purity. Double distilled water was used in all experiments. 99mTc was eluted from 99Mo/99mTc generator (RPF, Egyptian Atomic Energy Authority, Egypt). Radioactivity was measured by using A NaI (Tl) γ-ray scintillation counter (Scaler Ratemeter SR7 model, the United Kingdom). Bomem MB series-157(ABB, Canada) was used for IR spectra recording. The NMR spectral measurements were performed on a BrukerAvance (III) 400 MHz (BrukerAvance IVDR, Switzerland).
Synthesis of 6-amino-5-[(bis-(2-hydroxy-ethyl)-amino]methyl]2-methyl pyrimidin-4-ol (2)
A solution of (1.25 g, 0.01 mol) 6-amino-2-methylpyrimidin-4-ol 1 in 5 mL absolute ethanol was added with stirring to a hot mixture of (0.32 g, 0.0034 mol) paraformaldehyde dissolved in 7 mL of absolute ethanol and (0.0388 mol) of diethanolamine. Reflux for 24 h followed by the addition of glacial acetic acid. Crystals was obtained from water, with a good yield of 87%. The synthetic route for the target compound 2 is illustrated in Scheme 1.
The compound was produced in a reasonable yield and its structure was confirmed with spectral and elemental analysis. IR (KBr, cm−1): 3423, 3240, 2889, 1649; 1H-NMR (δ ppm): 10.98 (s, 1H, OH aromatic), 6.40 (s, 2H, NH2), 4.11 (m, 2H, OH aliphatic), 3.62 (s, 2H, Ar–CH2–N), 3.38 (m, 4H, –N–CH2–CH2), 2.50 (m, 4H, N–CH2), 2.17 (s, 3H, CH3).13C-NMR (δ ppm): 175.2 (C–OH), 160(NH2–C), 155.5 (C–CH3), 109 (=C–CH2), 59.5(N–CH2–CH2), 59.5(CH2–CH2–OH), 51.5(Ar–CH2–N), 25(CH3). MS (EI) m/z (%):243 ([M+, 100) Anal. calcd. For C10H18N4O3: C, 49.52; H, 7.40; N, 23.19, Found: C, 49.58; H, 7.49; N, 23.12.
Radiolabeling of 6-amino-5-[(bis-(2-hydroxy-ethyl)-amino]methyl]2-methyl pyrimidin-4-ol with technetium-99m
Radiolabeling studies of 6-amino-5-[(bis-(2-hydroxy-ethyl)-amino]methyl]2-methyl pyrimidin-4-ol were done using SnCl2·2H2O as a reducing agent [34]. All reaction parameters were varied in order to obtain the best RCY (radiochemical yield); as follows: Sn(II) amount (10–60 µg), pH (3–8), substrate amounts (0.5–3 mg), and the time of reaction (5–60 min). In an evacuated vials: Freshly prepared 30 µL SnCl2·2H2O solution containing 30 µg Sn(II) was added to the reaction mixture of ligand (2 mg in 0.5 mL DMSO) and the pH was adjusted to 5 followed by the addition of [99mTc]NaTcO4 (185 MBq in 0.45 mL saline) for 20 min reaction time. All parameters were studied at room temperature (R.T.) and all used solutions were fully deoxygenated. The optimum RCY obtained was 92.3%.
Radiochemical analysis
PC (paper chromatography)
RCY was detected by using PC with two different mobile phases; acetone and water: ethanol: ammonia mixture (5:2:1) [35, 36]. In case of using acetone free [99mTc]TcO4− was migrate at Rf = 1.00 while colloid ([99mTc]RH-Tc) and [99mTc]Tc complex remained at Rf = 0.00 [37, 38].
In case of water: ethanol: ammonia mixture (5:2:1); free [99mTc]TcO4− and the radiolabelled compound migrate between Rf = 0.70–1.00 while colloid remained at Rf = 0.00.
RCY was calculated by applying the following equation:
HPLC (High Performance Liquid Chromatography)
The RCY was further confirmed by using HPLC with C18 column (250 × 4.6 mm, 5 μm). HPLC was used to determine the RCY of the new 99mTc-complex. 20 µL of the optimized reaction mixture was injected to the column which is eluted isocratically with methanol: water (1:1 by volume), with a flow rate of 1 mL/min (Fig. 2) at 254 nm. UV radio-chromatogram peak for the 99mTc-complex was appeared at a retention time of 4.8 min, which coinciding with the unlabelled compound UV-peak (4.7 min). RCY of 99mTc-complex was calculated via HPLC confirmed the results determined via PC technique. HPLC was performed using the same method to indicates the chromatogram of Re-pyrimidine derivative complex (Fig. 3). Re-pyrimidine derivative complex showed nearly the same HPLC retention time (4.82 min) as our newly radiosynthesized 99mTc-complex.
Determination of partition coefficient
Approximately, 2 mL octan-1-ol and 1.9 mL distilled H2O were added to 0.1 [99mTc]Tc-complex, followed by vortexing up to 5 min. After that the two phases were separated using 1976 g centrifuge. Samples were withdrawn from each phase and measured for the radioactivity. The lipophilicity was calculated and equal to 1.29.
Determination of plasma protein binding of the radio-conjugate
[99mTc]Tc-complex was diluted using PBS at pH 7.4 to prevent radiolysis. Ascorbic acid (120 μg) was added to this diluted complex solution. Then, 25 μL from the diluted complex was added to 250 μL human plasma and incubated for 30 min at 37 °C. Sample was undergo ultrafiltration then measured in a γ-counter and compared with a control using PBS at pH 7.4 [39]. [99mTc]Tc-complex showed high affinity to human plasma (> 90%).
Cellular uptake study of [99mTc]Tc-pyrimidine derivative complex
The cellular uptake was carried out using previously protocols [40, 41]. The cell uptake of [99mTc]Tc-pyrimidine derivative complex was carried out on human breast cancer cell lines MCF-7. Firstly, MCF-7 cells (0.5 mL/well containing 4 × 105 cells) were seeded in 24-well culture plates and incubated for 24 h. In one well, 10μL of [99mTc]Tc-pyrimidine derivative complex was added, while standard 99mTc was added to the second well, then incubation at 37 °C for 15, 30, 60, and 120 min was attained. The supernatants were removed, and cells were washed using ice-cold PBS. Then, the cells were lysed using 0.1 N NaOH. The radioactivity of cell lysates was determined using γ-counter. The cell uptake was expressed as % of the total added activity and the cellular uptakes of the samples were analyzed against standards.
Rhenium complexation with the synthesized pyrimidine derivative
Into a 50 mL two-necked round-bottom flask fitted with a reflux condenser and containing 0.002 mol of the synthesized pyrimidine derivative with sodium borohydride (0.002 mol) were added. The content was heated at reflux for 0.5 h then 0.001 mol sodium perrhenate was added to the flask then reflux overnight. The reaction was monitored using thin layer chromatography (TLC) with mobile phase chloroform: methanol (9.6:0.4). After the completion of the reaction the product was purified using TLC yielding 61%. 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 11.21 (2OH, alcoholic), 6.41 (2NH2, amine), 3.71 (s, 4H, 2Ar–CH2), 3.63 (m, 8H, 2CH2–CH2–O–), 2.59 (m, 8H, 2CH2–CH2–O–), 2.1 (S, 6H, 2CH3). 13C-NMR (300 MHz, DMSO-d6) δ (ppm): 176.5 (2[C–OH]), 161 (2[N=C–NH2]), 154.7 (2[C–CH3]), 108.2 (C–CH2–N), 52.5 (4[N–CH2CH2–O–]), 50.7 (2[Ar–CH2], 33.5 (4[N–CH2CH2–O–]). MS (m/z): 699.
Molecular docking studies
Molecular docking studies were performed using Discovery Studio 2016, The X-ray crystal structure of CDK2 (PDB ID: 4FX3) co-crystallized with oxoindole was downloaded from the Protein Data Bank (www.rcsb.org). Moreover, there are adjustments for the protein preparation as addition of polar hydrogen, of side chains and removal of water molecules. The protein was kept rigid whereas the ligand was endorsed flexible through the docking simulation. For each compound, the top-score docking poses were chosen for final ligand target interaction analysis.
Biodistribution studies
This study was guided by the Egyptian Atomic Energy Authority animal ethics committee, which follows European Community guidelines [42]. Male Swiss albino mice (25–30 gm) were used. Mice were obtained from the local animal house at EAEA, Cairo, Egypt. From female mouse (donor), Ehrlich ascites carcinoma was withdrawn, and then the solution was injected intramuscularly in the left thigh to produce solid tumor after 6–7 days. [99mTc]Tc-complex was injected intravenously and the mice were anaesthetized, weighted and sacrificed at different time intervals (10, 45, 75 and 120 min). Organs were separated and weighted (muscles, bone and blood were taken as 40, 10 and 7%, respectively) [25]. All results were calculated as %ID/g organ.
Blocking study
A pre-injection of unlabeled synthesized compound (pyrimidine derivative) was done followed by the injection of [99mTc]Tc-pyrimidine derivative complex after 10 min and the radioactivities were assayed.
Results and discussion
Characterization of 6-amino-5-[(bis-(2-hydroxy-ethyl)-amino]methyl]2-methyl pyrimidin-4-ol
The new pyrimidine derivative was synthesized according to the reaction depicted in Scheme 1. The starting 2-methyl-4-amino-6-hydroxypyrimidine 1 was synthesized following a literature known procedure [43] using acetamide hydrochloride and cyanoacetic ester in the presence of sodium methoxide in methanol as a condensing agent in a one-pot reaction to afford compound 2.
Radiolabeling of 6-amino-5-[(bis-(2-hydroxy-ethyl)-amino]methyl]2-methyl pyrimidin-4-ol with technetium-99m
Figure 4 showed the variation of %RCY according to reaction parameters. The optimum conditions to obtain the maximum RCY of 92.3% were 30 µg Sn(II) and 2 mg substrate at pH 5 for 20 min at R.T.
Stannous ions acted as the best reducing agent for most of Tc-radiopharmaceutical kits, this reducing agent reduces technetium + 7 to the desired oxidation state [44, 45]. Sn(II) amount (10 µg) showed low RCY (40.32%) that can be due to the reduction of [99mTc]TcO4− was not completed [46]; while at higher Sn(II) content more than 30 µg, RCY was decreased due to the formation of undesired products (colloid) [25, 47]. At acidic pH (pH 3), the %colloid with low (4.40%) while the %free [99mTc]TcO4− was high (42.10%) and vice versa at high pH value (at pH 8, colloid was 34.25% and free TcO4− was 2.22%). Substrate amount effect on the %RCY, the lower contents of substrate the lower RCY that may be due to the substrate amount was insufficient to chelate with all the reduced 99mTc. Increasing the amount of substrate more than the optimum value (2 mg) has no significant changes in RCY. The complexation process was completed rapidly, after 20 min and still stable for more than 6 h.
Serum in-vitro stability
Human serum stability was checked using radiochemical purity (RCP) determination using aforementioned PC technique at different time (0, 3, 6, 9,12, 15, 18, 21 and 24 h). Figure 5 shows that 99mTc-complex was highly stable in serum, and %RCP was decreased only by 3% after four half-lives. This propose that the new complex would show prolonged stability for in-vivo study.
Cytotoxicity assay
The breast cancer cell line (MCF-7) was used to determine the inhibitory effect of compounds on cell growth using the MTT assay according to previously well-known method. The IC50 of synthesized pyrimidine derivative on breast cancer cell line (MCF-7) were tested. The result indicated IC50 = 7.22 µM (Fig. 6), which indicated its strong activity against the breast cancer cell line.
Cellular uptake study of [99mTc]Tc-pyrimidine derivative complex
Cellular uptake, % reached 11.32% at 60 min with no changes in uptake level at 120 min; while the control ratio showed 0.98% at all tested time points. This result indicates that [99mTc]Tc-pyrimidine derivative complex could bind quickly to the breast cancer cells (MCF-7).
Complexation of the new synthesized pyrimidine derivative with Re as 99mTc analogue
The new pyrimidine derivative was complexed with Re according to the reaction in Scheme 2. The synthesized compound was complexed with rhenium via one step method. The similar coordination chemistry between technetium-99m and rhenium can lead us to detect the complex structure of technetium-99m with the new synthesized pyrimidine derivative. Two molecules of the compound were chelated with technetium-99m via tow oxygen atoms from each molecule.
Docking studies
Computational tools have become essential part for the design and development of therapeutically effective novel chemical entity [48, 49]. To gain further evidence for obtaining a good cell cycle inhibitor, a molecular docking study was carried out on the 3D structure of CDK2 enzyme using Discovery Studio. The enzyme used in this study was obtained from protein data bank (PDB ID: 4FX3) [50], the structure checked for missed atoms and bonds. Water molecules and all residues other than ligands were manually deleted and the structures were refined as follow. The binding modes in the ATP binding site of CDK2 have already been reported and involving key binding residues, Lys 33, Phe 80, Glu 81, Leu 83, Hist 84, Asp 86, Lys 89 and Asp 145 [51]. All potent CDK inhibitors act by competing with ATP or blocking ATP binding based on the interaction with the catalytic site of CDK. From the docking results, the ligand compound illustrated interaction with the active site forming three hydrogen bonds with Lys 33, Asp 145 and Asp 86. Additionally, it formed Van der Waal interaction with amino acids Phe 80, Leu 83, Lys 89 and Hist 84 and Pi-anion interaction with amino acid Asp 86 (Figs. 7, 8 and 9). Docking of compound 9 into CDK 2 active site revealed several molecular interactions (hydrogen bond, π interaction and hydrophobic interactions) that were considered responsible for the observed affinity of the compound. It showed 7 H-bond interactions, three H-bonds with Gln 131, two H- bonds with Asp 86, one H-bond with Ile 10 and one H-hydrogen bond with Asn 132. Furthermore, it showed pi-alkyl interaction with Val 18, Val 64, Phe 80, Asp 145 and Ala 144. The docked complexes showed characteristic hydrogen bonding interactions with crucial residues of the active-site, such as Lys 33, Asp 145. Regarding proposed complex 1, the compound formed eight hydrogen bonds, 2 hydrogen bonds with Asp 86, 2 H-bonds with Gln 131, 2 H-bonds ASN 132, one H-bond with ASN 132 and one H-bond with Lys 33. Moreover, it formed Vander Waal interaction with Val 64, Leu 55 and Leu 134. Also, it formed Pi-alkyl interaction with amino acids Phe 80, Ala 144 and Val 18. Additionally, it formed attractive charge interaction with Asp 86. Furthermore, proposed complex 2 showed interaction with eleven hydrogen bond interactions, four hydrogen bonds with Asp 86, three hydrogen bonds with Gln 131, two hydrogen bonds with Ile 10, one hydrogen bond with Gly 11 and one hydrogen bond with Asn 133. Also, it formed Van der Waal interaction with Leu 133, Leu 83, Asp 145, Lue 33 and Val 64. It formed attractive charge interaction with Asp 86 and Leu 134, Ala 31, Val 18 and Phe 80 and pi-alkyl interaction. For proposed complex 3, it formed eight H-bond interaction, one H-bonds with Asp86, two H-bonds with His 84, two H-bonds Asn 132, one H-bond Gln 85, one hydrogen bond with Asp 133 and one H-bond Lys 33. Moreover, it formed Van der Waal interaction with essential amino acid residues such as Gln 12, Leu 285, Phe 146, Glu 51, Leu 134, Gln 131 and pi-alkyl interaction with Leu 55, Val 64, Leu 134, Ala 144, Val 18 and Phe 80. Additionally, it formed pi-cation interaction with Phe 80.
The synthesized compound was making a complex with technetium-99m via direct labeling technique through the presence of donor atoms. By using energy minimization study the most probable complex of the compound with technetium-99m was presented in the following figure (Fig. 10). Two molecules of the compound were chelated with technetium-99m via tow oxygen atoms from each molecule.
Biodistribution studies
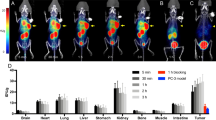
The biodistribution of [99mTc]Tc-pyrimidine derivative complex was performed in solid tumor-bearing mice at 10-, 45-, 75- and 120-min post injection. Distribution of [99mTc]Tc-pyrimidine derivative complex in the body organs was shown in Fig. 11. The accumulation of [99mTc]Tc-pyrimidine derivative complex was observed in a tumor site, showing 2.03, 4.21 and 3.89% ID/g at 45, 75 and 120 min, respectively. This complex was mainly excreted via both hepatobiliary and urinary pathways. Intestine showed 6.19% ID/g at 120 min and kidneys showed 3.25% ID/g at 120 min. The tumor/normal tissue ratio showed its high value of 6.58 at 75 and 120 min (Fig. 12), While the tumor/blood ratio showed its high value of 2.53 at 120 min (Fig. 13). The aforementioned promising data offers a promising imaging agent for solid tumor.
Blocking study
Blocking study was done to confirm the specificity binding of radioligand to the tumor site. The tumor uptake was reached its highest value of 2.01% ID/g showing its highest T/NT of 3.41 at the same time (Fig. 14). The blocking study was compared with the aforementioned results of biodistribution and it confirmed that the tumor uptake in case of blocking study was less than one half in comparison with the unblocked radioligand. These data confirm that the uptake of [99mTc]Tc-pyrimidine derivative complex was specific to tumor cells.
Conclusion
A novel pyrimidine derivative was well synthesized and characterized via various techniques and then radiolabeled with technetium-99m with a high radiochemical yield of 92.3% and good in vitro stability at the optimum conditions (30 µg Sn(II) and 2 mg substrate at pH 5 for 20 min at R.T.). Docking of the synthesized compound revealed that it fit well into the binding-site and display favorable interactions with the crucial amino acid residues. Biodistribution study indicates effective accumulation of the new synthesized complex ([99mTc]Tc-6-amino-5-[(bis-(2-hydroxy-ethyl)-amino]methyl]2-methyl pyrimidin-4-ol complex) and offers a good candidate for cancer imaging after more preclinical and clinical studies.
References
Hassan RM, Abd-Allah WH, Salman AM, El-Azzouny AA-S, Aboul-Enein MN (2019) Design, synthesis and anticancer evaluation of novel 1,3-benzodioxoles and 1,4-benzodioxines. Eur J Pharm Sci 139:105045
Selim AA, Essa BM, Abdelmonem IM, Amin MA, Sarhan MO (2021) Extraction, purification and radioiodination of Khellin as cancer theranostic agent. Appl Radiat Isot 178:109970
Vineis P, Wild CP (2014) Global cancer patterns: causes and prevention. The Lancet 383:549–557
Harper J, Adams P (2001) Cyclin-dependent kinases. Chem Rev 101:2511–2526
Kiyota A, Shintani S, Mihara M, Nakahara Y, Ueyama Y, Matsumura T, Tachikawa T, Wong DT (2002) Anti-epidermal growth factor receptor monoclonal antibody 225 upregulates p27KIP1 and p15INK4B and induces G1 arrest in oral squamous carcinoma cell lines. Oncology 63:92–98
Jain K, Chitre T, Miniyar P, Kathiravan M, Bendre V, Veer V, Shahane S, Shishoo C (2006) Biological and medicinal significance of pyrimidines. Curr Sci 793–803
Heidelberger C, Chaudhuri N, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer R, Pleven E, Scheiner J (1957) Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 179:663–666
Giller S, Zhuk R, Lidak M (1967) Analogs of pyrimidine nucleosides. I/ N1-(alpha-furanidyl) derivatives of natural pyrimidine bases and their antimetabolities. Dokl Akad Nauk SSSR 176:332
Kennedy B, Torkelson JL, Torlakovic E (1999) Uracil mustard revisited. Cancer 85:2265–2272
Society N-OWGotGC (2003) Neuro-oncology working group 01 trial of nimustine plus teniposide versus nimustine plus cytarabine chemotherapy in addition to involved-field radiotherapy in the first-line treatment of malignant glioma. J Clin Oncol 21:3276–3284
Walko CM, Lindley C (2005) Capecitabine: a review. Clin Ther 27:23–44
Katritzky A, Rees C, Scriven E (1996) Comprehensive Heterocyclic Chemistry II, Boulton, AJ, Ed., 6. Pergamon Press, Oxford–New York–Tokyo
Scott EN, Thomas AL, Molife LR, Ahmed S, Blagden S, Fong PC, Kowal K, McCoy C, Wiesinger H, Steward W (2009) A phase I dose escalation study of the pharmacokinetics and tolerability of ZK 304709, an oral multi-targeted growth inhibitor (MTGI™), in patients with advanced solid tumours. Cancer Chemother Pharmacol 64:425–429
Scholz A, Wagner K, Welzel M, Remlinger F, Wiedenmann B, Siemeister G, Rosewicz S, Detjen KM (2009) The oral multitarget tumour growth inhibitor, ZK 304709, inhibits growth of pancreatic neuroendocrine tumours in an orthotopic mouse model. Gut 58:261–270
Zhou W, Huang A, Zhang Y, Lin Q, Guo W, You Z, Yi Z, Liu M, Chen Y (2015) Design and optimization of hybrid of 2,4-diaminopyrimidine and arylthiazole scaffold as anticancer cell proliferation and migration agents. Eur J Med Chem 96:269–280
Corner J, Bailey CD (2009) Cancer nursing: care in context. John Wiley & Sons, New York
Gabriel JA (2007) The biology of cancer. Wiley Online Library, West Sussex
Lin J, Qiu L, Lv G, Li K, Wang W, Liu G, Zhao X, Wang S (2017) Synthesis and preliminary biological evaluation of a 99mTc-chlorambucil derivative as a potential tumor imaging agent. J Label Compd Radiopharm 60:116–123
Yarbro CH, Wujcik D, Gobel BH (2010) Cancer nursing: principles and practice. Jones & Bartlett Publishers
Sakr T, El-Safoury D, Awad GA, Motaleb M (2013) Biodistribution of 99mTc-sunitinib as a potential radiotracer for tumor hypoxia imaging. J Label Compd Radiopharm 56:392–395
Guy C, Ffytche D (2005) An introduction to the principles of medical imaging. Imperial College Press London
Hendee WR, Ritenour ER (2003) Medical imaging physics. John Wiley & Sons, New York
Jurisson SS, Lydon JD (1999) Potential technetium small molecule radiopharmaceuticals. Chem Rev 99:2205–2218
Méndez-Rojas MA, Kharisov BI, Tsivadze AY (2006) Recent advances on technetium complexes: coordination chemistry and medical applications. J Coord Chem 59:1–63
Motaleb M, Selim AA, El-Tawoosy M, Sanad M, El-Hashash M (2017) Synthesis, radiolabeling and biological distribution of a new dioxime derivative as a potential tumor imaging agent. J Radioanal Nucl Chem 314:1517–1522
Essa BM, Selim AA, Sayed GH, Anwer KE (2022) Conventional and microwave-assisted synthesis, anticancer evaluation, 99mTc-coupling and In-vivo study of some novel pyrazolone derivatives. Bioorg Chem 125:105846
Engelhardt EL, Schneider RF, Seeholzer SH, Stobbe CC, Chapman JD (2002) The synthesis and radiolabeling of 2-nitroimidazole derivatives of cyclam and their preclinical evaluation as positive markers of tumor hypoxia. J Nucl Med 43:837–850
Wang J, Yang J, Yan Z, Duan X, Tan C, Shen Y, Wu W (2011) Synthesis and preliminary biological evaluation of [99mTc (CO) 3 (IDA–PEG3–CB)]− for tumor imaging. J Radioanal Nucl Chem 287:465–469
Sun X, Chu T, Wang X (2010) Preliminary studies of 99mTc-BnAO and its analogues: synthesis, radiolabeling and in vitro cell uptake. Nucl Med Biol 37:117–123
Mallia MB, Subramanian S, Mathur A, Sarma H, Venkatesh M, Banerjee S (2010) Synthesis and evaluation of 2-, 4-, 5-substituted nitroimidazole-iminodiacetic acid-99mTc (CO) 3 complexes to target hypoxic tumors. J Label Compd Radiopharm 53:535–542
Satpati D, Korde A, Venkatesh M, Banerjee S (2009) Preparation and bioevaluation of a 99mTc-labeled chlorambucil analog as a tumor targeting agent. Appl Radiat Isot 67:1644–1649
Riché F, du Moulinet DA, Sèpe S, Riou L, Fagret D, Vidal M (2001) Nitroimidazoles and hypoxia imaging: synthesis of three technetium-99m complexes bearing a nitroimidazole group: biological results. Bioorg Med Chem Lett 11:71–74
Sakr T, Essa B, El-Essawy F, El-Mohty A (2014) Synthesis and biodistribution of 99m Tc-PyDA as a potential marker for tumor hypoxia imaging. Radiochemistry 56:76–80
Essa B, Sakr T, Khedr MA, El-Essawy F, El-Mohty A (2015) 99mTc-amitrole as a novel selective imaging probe for solid tumor: in silico and preclinical pharmacological study. Eur J Pharm Sci 76:102–109
Essa BM, El-Mohty AA, El-Hashash MA, Sakr TM (2020) 99mTc-citrate-gold nanoparticles as a tumor tracer: synthesis, characterization, radiolabeling and in-vivo studies. Radiochim Acta 108:809–819
Sakr TM, El-Hashash M, El-Mohty A, Essa BM (2020) 99mTc-gallic-gold nanoparticles as a new imaging platform for tumor targeting. Appl Radiat Isot 164:109269
Ebrahem EM, Sayed GH, Gad GN, Anwer KE, Selim AA (2022) Histopathology, pharmacokinetics and estimation of interleukin-6 levels of Moringa oleifera leaves extract-functionalized selenium nanoparticles against rats induced hepatocellular carcinoma. Cancer Nanotechnol 13:1–26
Fayez H, Selim AA (2022) Bone targeted new zoledronate derivative: design, synthesis, 99mTc-coupling, in-silico study and preclinical evaluation for promising osteosarcoma therapy. Int J Radiat Biol 1–34
Müller C, Farkas R, Borgna F, Schmid RM, Benesova M, Schibli R (2017) Synthesis, radiolabeling, and characterization of plasma protein-binding ligands: potential tools for modulation of the pharmacokinetic properties of (radio) pharmaceuticals. Bioconjug Chem 28:2372–2383
Daruwati I, Gwiharto AK, Kurniawan A, Mahendra I, Achmad TH, Syaifudin M, Muchtaridi M (2021) Synthesis, stability, and cellular uptake of 131I-estradiol against MCF7 and T-47D human cell lines as a radioligand for binding assay. Heliyon 7:e08438
Huang H, Li K, Lv G, Liu G, Zhao X, Liu Q, Wang S, Li X, Qiu L, Lin J (2018) One-step 18F-labeling of estradiol derivative for PET imaging of breast cancer. Contrast Media Mol Imaging 2018
Abdulwahab HG, Harras MF, El Menofy NG, Hegab AM, Essa BM, Selim AA, Sakr TM, El-Zahabi HS (2020) Novel thiobarbiturates as potent urease inhibitors with potential antibacterial activity: design, synthesis, radiolabeling and biodistribution study. Bioorg Med Chem 28:115759
Maggiolo A, Phillips AP, Hitchings GH (1951) Synthesis of 2-Methyl-4-amino-6-substituted Aminopyrimidines1. J Am Chem Soc 73:106–107
Motaleb M, Selim AA, El-Tawoosy M, Sanad M, El-Hashash M (2018) Synthesis, characterization, radiolabeling and biodistribution of a novel cyclohexane dioxime derivative as a potential candidate for tumor imaging. Int J Radiat Biol 94:590–596
Owunwanne A, Marinsky J, Blau M (1977) Charge and nature of technetium species produced in the reduction of pertechnetate by stannous ion. J Nucl Med 18:1099
Sakr TM, Khedr MA, Rashed HM, Mohamed ME (2018) In silico-based repositioning of phosphinothricin as a novel technetium-99m imaging probe with potential anti-cancer activity. Molecules 23:496
Motaleb M, Sanad M, Selim A, El-Tawoosy M, Abd-Allah M (2018) Synthesis, characterization, and radiolabeling of heterocyclic bisphosphonate derivative as a potential agent for bone imaging. Radiochemistry 60:201–207
Podlogar B, Muegge I, Brice L (2001) Computational methods to estimate drug development parameters. Curr Opin Drug Discov Dev 4:102
Cherukupalli S, Chandrasekaran B, Kryštof V, Aleti RR, Sayyad N, Merugu SR, Kushwaha ND, Karpoormath R (2018) Synthesis, anticancer evaluation, and molecular docking studies of some novel 4, 6-disubstituted pyrazolo [3, 4-d] pyrimidines as cyclin-dependent kinase 2 (CDK2) inhibitors. Bioorg Chem 79:46–59
Al-Warhi T, El Kerdawy AM, Aljaeed N, Ismael OE, Ayyad RR, Eldehna WM, Abdel-Aziz HA, Al-Ansary GH (2020) Synthesis, biological evaluation and in silico studies of certain oxindole-indole conjugates as anticancer CDK inhibitors. Molecules 25:2031
Li Y, Gao W, Li F, Wang J, Zhang J, Yang Y, Zhang S, Yang L (2013) An in silico exploration of the interaction mechanism of pyrazolo [1, 5-a] pyrimidine type CDK2 inhibitors. Mol BioSyst 9:2266–2281
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Essa, B.M., Abd-Allah, W.H. & Sakr, T.M. Synthesis, 99mTc-labeling, in-vivo study and in-silico investigation of 6-amino-5-[(bis-(2-hydroxy-ethyl)-amino]methyl]2-methyl pyrimidin-4-ol as a potential probe for tumor targeting. J Radioanal Nucl Chem 331, 3601–3612 (2022). https://doi.org/10.1007/s10967-022-08412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08412-z