Abstract
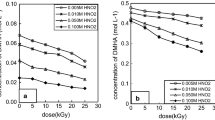
Dihydroxyurea (DHU) is a new salt-free reducing agent applied for the separation of Pu and Np from U in spent fuel reprocessing. This paper reports on the γ-ray radiolysis of DHU in HNO3 and its radiolytic products. DHU is relatively stable against radiation at the dose supposed to be absorbed in the separation of Pu and Np from U. The main radiolytic products are H2, CO2, N2O and \({\text{NH}}_{{4}}^{ + }\). The volume fraction of H2 increases with the dose, but decreases with increasing HNO3 concentration. The quantity of CO2, N2O and \({\text{NH}}_{{4}}^{ + }\) increases with the HNO3 concentration and the dose.






Similar content being viewed by others
References
Natarajan R (2017) Reprocessing of spent nuclear fuel in India: present challenges and future programme. Prog Nucl Energy 101:118–132
Choppin GR, Morgenstern AJ (2000) Radionuclide separations in radioactive waste disposal. J Radioanal Nucl Chem 243:45–51
Schlea CS, Caverly MR, Henry HE, et al. (1963) Uranium (IV) nitrate as a reducing agent for plutonium (IV) in the Purex process. DP-808:1–20
Sze YK, Clegg LJ, Gerwing AF et al (1982) Oxidation of Pu (III) by nitric acid in tri-n-butyl phosphate solutions. Part I. Kinetics of the reaction and its effect on plutonium losses in countercurrent liquid-liquid extraction. Nucl Technol 56:527–534
Sze YK, Gosselin JA (1983) Oxidation of Pu (III) by nitric acid in tri-n-butyl phosphate solutions. Part II. Chemical methods for the suppression of oxidation to improve plutonium separation in contactor operation. Nucl Technol 63:431–441
McKibben JM, Bercaw JE (1971) Hydroxylamine nitrate as a plutonium reductant in the purex solvent extraction process. No. DP-1248:1–22
Zheng WF, Zhang ZF, Lin ZJ et al (2001) Study on separation of Np from U by acetohydroxamic acid in purex process. Chin J Nucl Sci Eng 21:369–374 ((in Chinese))
Ochsenfeld W, Petrich G, Schmidt HJ et al (1983) Neptunium decontamination in a uranium purification cycle of a spent fuel reprocessing plant. Sep Sci Technol 18:1685–1698
Biddle P, Miles JH (1968) Rate of reaction of nitrous acid with hydrazine and with sulphamic acid: its application to nitrous acid control in two-phase industrial systems. J Inorg Nucl Chem 30:1291–1297
Ye GA (2004) Application analysis of organic salt-free reagents in Purex process. Natl Ann Conf Nuclear Chem Chem Eng 02:152–158 ((in Chinese))
Taylor RJ, May I, Koltunov VS et al (1998) Kinetic and solvent extraction studies of the selective reduction of Np (VI) by new salt-free reducing agents. Radiochim Acta 81:149–156
Ye GA (2004) Review on the study and application of organic salt-free reagent in purex process. Atom Energy Sci Technol 38:152–158 ((in Chinese))
Wang JH, Cao XJ, Li C et al (2015) Effect of HNO3 on the γ radiolysis and radiolytic liquid products of N, N-Dimethylhydroxylamine. Acta Phys Chim Sin 31:559–565
Zhang AY, Hu JX, Zhang XY et al (2001) Hydroxylamine derivative in PUREX process. VI. Study on the partition of uranium/neptunium and uranium/plutonium with N, N-diethylhydroxylamine in the purification cycle of uranium contactor. Solvent Extr Ion Exch 19:965–979
Miles JH (1989) Stabilization of an element against oxidation. UK Patent: 2216886
Zhu ZW (2003) Study on the application of hydroxyurea-a salt-free agents in the Purex process. China Institute of Atomic Energy (in Chinese)
Wang JL, Yan TH (2020) Development of distribution behavior of technetium in purex process. J Nuclear Radiochem 42:10–19 ((in Chinese))
Li Q (2013)γRadiation Stability and Radiolysis Products of Acetohydroxamic Acid and Dihydroxyurea. Shanghai University (in Chinese)
Yan TH, Zhen WF, Ye GA et al (2009) Synthesis of dihydroxyurea and its application to the U/Pu split in the PUREX process. J Radioanal Nucl Chem 279:293–299
Fishbein WN (1968) A simple, sensitive, and specific colorimetric assay for dihydroxyurea. Anal Chim Acta 40:269–275
Zhang B, Wang JH, Bao BR et al (2006) Qualitative and quantitative analysis of hydrogen and carbon monoxide produced from radiolysis of acetohydroxamic acid in aqueous solution. J Shanghai Univ 12:408–411 (in Chinese)
Chen CH, Wu YB (2004) Determination of ammonium by ion chromatography for spectrophotometry. Modern Sci Instrum 5:64–65 (in Chinese)
Editorial board of water and wastewater detection and analysis methods by State Environmental Protection Administration (2002) In: Water and wastewater monitoring and analysis methods, 4rd edn. China Environment publishing house, Beijing
Li HB, Su Zh, Cong HF et al (2012) α and γ irradiation stability of 30% TBP-Kerosene-HNO3 systems. J Nucl Radiochem 34:281–285 (in Chinese)
Karraker DG (2002) Radiation chemistry of acetohydroxamic acid in the Urex process, WSRC-TR-2002–00283:1–8
Le CS (2011) Water radiolysis: influence of oxide surfaces on H2 production under ionizing radiation. Water 3:235–253
Spinks JWT, Woods RJ (1976) An introduction to radiation chemistry, 2nd edn. A Wiley-Interscience Publication. John Wiley & Sons, New York, pp 247–295
Jiang PY, Nagaishi R, Yotsuyanagi T et al (1994) γ-Radiolysis study of concentrated nitric acid solutions. J Chem Soc Faraday Trans 90:93–95
Balcerzyk AE et al (2012) Picosecond pulse radiolysis study of highly concentrated nitric acid solutions: formation mechanism of NO3· radical. J Phys Chem 116:7302–7307
Katsumura Y, Jiang PY, Nagaishi R et al (1991) Pulse radiolysis study of aqueous nitric acid solutions: formation mechanism, yield, and reactivity of NO3 radical. J Phys Chem 95:4435–4439
King S (2003) The nitric oxide producing reactions of hydroxyurea. Curr Med Chem 10:437–452
Ohno K, Kishimoto N, Iwamoto T, et al (2017) Global exploration of isomers and isomerization channels on the quantum chemical potential energy surface of H3CNO3. J Comput Chem, 1–19
Lefort M, Tarrago X (1961) Significance and function of NH2 in the ionizing radiolysis of hydroxylamine aqueous solution. J Inorg Nucl Chem 16:169–189
Simic M, Hayon E (1971) Intermediates produced from the one-electron oxidation and reduction of hydroxylamines. Acid-base properties of the amino, hydroxyamino, and methoxyamino radicals. J Amer Chem Soc 93(23):5982–5986
Miranda KM (2005) The chemistry of nitroxyl (HNO) and implications in biology. Coord Chem Rev 249:433–455
Miranda KM, Paolocci N, Katori T et al (2003) A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc Natl Acad Sci USA 100:9196–9201
Bonner FT, Hughes MN (1989) The aqueous solution chemistry of nitrogen in low positive oxidation states. ChemInform 7:215–234
Cheng YG (1992) hydroxylamine and salt. Fire Explos 6:52–57 (in Chinese)
Horne GP, Grimes TS, Bauer WF et al (2019) Effect of ionizing radiation on the redox chemistry of penta-and hexavalent Americium. Inorg Chem 58:8551–8559
Nagaishi R (2001) A model for radiolysis of nitric acid and its application to the radiation chemistry of uranium ion in nitric acid medium. Radiat Phys Chem 60:369–375
Horne GP, Gregson CR, Sims HE et al (2017) Plutonium and americium alpha radiolysis of nitric acid solutions. J Phys Chem B 121:883–889
Kazanjian AR, Miner FJ, Brown AK et al (1970) Radiolysis of nitric acid solution: l.e.t. effects. Trans Faraday Soc 66:2192–2198
Mincher BJ, Precek M, Mezyk SP et al (2013) The redox chemistry of neptunium in γ-irradiated aqueous nitric acid. Radiochim Acta 101:259–266
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, X.Y., Wang, J.H., Li, Q. et al. γ-ray radiolysis of dihydroxyurea in nitric acid and its radiolytic products. J Radioanal Nucl Chem 331, 3517–3524 (2022). https://doi.org/10.1007/s10967-022-08388-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08388-w