Abstract
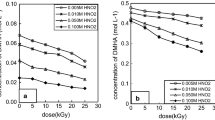
Dihydroxyurea (DHU) is a novel salt-free reductant used in the reprocessing of spent fuel. This paper reports on the radiolysis of DHU in water and its radiolytic products. The concentration reduction ratio of DHU after irradiation increases with the dose but decreases with increasing DHU concentration. The radiolytic by-products are H2, CO2 NH4+ and HNO2. The amount of H2, CO2 and NH4+ increases with DHU concentration, but that of HNO2 decreases with increasing DHU concentration. The quantity of CO2 and NH4+ increases with the dose. The radiation chemical yields of DHU destruction and radiolytic products are determined.







Similar content being viewed by others
Data availability
Data that support the findings of this study are available within the attached files.
References
Adamantiades A, Kessides I (2009) Nuclear power for sustainable development: current status and future prospects. Energy Policy 37:5149–5166
World nuclear association. Processing of used nuclear fuel. https://www.world-nuclear.org/information-library/nuclear-fuel-cycle/fuel-recycling/processing-of-used-nuclear-fuel. Aspx
Sun XZh, Luo ZhH (2016) Status of global spent fuel reprocessing. Nucl Saf 15(2):13–16 (in Chinese)
Silverio LB, Lamas WQ (2011) An analysis of development and research on spent nuclear fuel reprocessing. Energy Policy 39:281–289
Wang JH, Li Q, Wu MH, Xu G, Li Ch, Bao BR, Zheng WF, He H, Zhang ShD (2012) Radiolysis of N, N-dimethylhydroxylamine and its radiolytic liquid organics. J Radioanal Nucl Chem 292:249–254
Benedict M, Pigford TH, Levi HW (1981) Nuclear chemical engineering. McGraw-Hill Book Company
Choppin GR (1999) Future of separation science in U.S. department of energy processing and remediation. In: ACS Symposium. 716, pp 13-19
Schlea CS, Caverly MR, Henry HE, Jenkons WJ (1963) Uranium(IV) nitrate as a reducing agent for plutonium(IV) in the purex process. DP-808, Chemical separations processes for plutonium and uranium (TID-4500, 21st Ed.), pp 1–20
Mckibben JM, Bercaw JE (1971) Hydroxylamine nitrate as a plutonium reductant in the PUREX solvent extraction process. DP-1248, Chemistry separations processes for plutonium and uranium (TID-4500, Uc-10), pp 1–22
Sze YK, Clegg LJ, Gerwing AF, Grant GR (1982) Oxidation of Pu(III) by nitric acid in tri-n-butyl phosphate solutions. Part I. Kinetics of the reaction and its effect on plutonium losses in countercurrent liquid–liquid extraction. J Nucl Technol 56:527–534
Sze YK, Gosselin JA (1983) Oxidation of Pu(III) by nitric acid in tri-n-butyl phosphate solutions. Part II. Chemical methods for the suppression of oxidation to improve plutonium separation in contactor operation. J Nucl Technol 63:431–441
Biddle P, McKAY HAC, Miles JH (1965) The role of nitrous acid in the reduction of plutonium(IV) by uranium in the TBP system. Solvent extraction chemistry of metals. In: Proceedings of international conference on solvent extraction of metals. Harwell, Macmillan, London, pp 133–154
Ochsenfeld W, Petrich G, Schmidt HJ, Stollenwerk AH, Wiese H (1983) Neptunium decontamination in a uranium purification cycle of a spent fuel reprocessing plant. Separ Sci Technol 18:1685–1698
Biddle P, Miles JH (1968) Rate of reaction of nitrous acid with hydrazine and with sulphamic acid. J Inorg Nucl Chem 30:1291–1297
Zheng WF, Zhang ZF, Lin ZJ, Chang ZhY, Zhu JM, Zhu ZhW (2001) Study on separation of Np from U by acetohydroxamic acid in purex process. Chin J Nucl Sci Eng 21(4):369–374 (in Chinese)
Ye GA (2004) Review on the study and application of organic salt-free reagent in purex process. At Energy Sci Technol 38(2):152–158 (in Chinese)
Taylor RJ, May I, Koltunov VS, Baranov SM, Marchenko VI, Mezhov EA, Pastuschak VG, Zhuravleva GI, Savilova OA (1998) Kinetic and solvent extraction studies of the selective reduction of Np (VI) by new salt-free reducing agents. Radiochim Acta 81:149–156
Miles JH (1989) Stabilisation of an element against oxidation during a solvent extraction process. UK Patent 2216886, 1989.10
Xiao ST, Ye GA, Liu XCh, Luo FX, Lan T, Li FF (2011) Kinetics of reaction between Pu(IV) and hydroxysemicarbazide in nitric acid solution. At Energy Sci Technol 45(3):277–281
Wang Y, Xiao ST, Lan T, Liu XCh, OuYang YG, Li HB (2018) Kinetics of reaction between Np(VI) and hydroxysemicarbazide in nitric acid solution. J Nucl RadioChem 40(6):359–365
Xiao ST, Ye GA, Liu XCh, Luo FX, Li HR, Li FF (2011) Kinetics of reaction between hydroxysemicarbazide and HNO2 in perchloric acid solution. At Energ Sci Technol 45(4):402–406
Zhu ZW, He JY, Zhang ZF, Zhang Yu, Zhu JM, Zhen WF (2004) Uranium/plutonium and uranium/neptunium separation by the Purex process using hydroxyurea. J Radioanal Nucl Chem 262(3):707–711
Zhu ZhW (2003) Study on the application of hydroxyurea—a salt free agent in the purex process. Doctorate dissertation, China Institute of Atomic Energy, pp 86–96 (in Chinese)
Yan TH, Zheng WF, Ye GA, Zhang Y, Xian L, Di Y, Bian XY (2009) Synthesis of dihydroxyurea and its application to the U/Pu split in the PUREX process. J Radioanal Nucl Chem 279:293–299
Yan TH, Zheng WF, Ye GA, Zhang Y, Xian L, Bian XY, Di Y (2009) Reaction kinetics of dihydroxyurea with nitrous acid and its effect on stabilizing Pu(III). J Nucl Radio Chem 31:65–71 (in Chinese)
Yan TH, Zheng WF, Zuo C, Xian L, Zhang Y, Bian XY, Li RX, Di Y (2010) The reduction of Np(VI) and Np(V) by tit dihydroxyurea and its application to the U/Np separation in the PUREX process. Radiochim Acta 98:35–38
Yan TH (2008) Studies on the application of dihydroxyurea in the Purex process. Doctoral dissertation, China Institute of Atomic Energy, pp 13
Wang JH, Zhang J, Li Q, Zhang Sh, Wu MH, Bao BR, Zheng WF, Yan TH, He H, Zhang ShD, Cao XJ (2014) New method of dihydroxyurea synthesis. CN Patent CN102659637 B
Zhang J (2012) Synthesis and purification of dihydroxyurea. Master dissertation, Shanghai University, pp 9–47
Li Q (2013) γ Radiation stability and radiolysis products of acetohydroxamic acid and dihydroxyurea. Master dissertation, Shanghai University, 9, 36–41
William NF (1968) A simple, sensitive, and specific colorimetric assay for dihydroxyurea. Anal Chim Acta 40:269–275
Wang JH, Wang ShX, Bao BR, Li Zh, Li Ch, Zheng WF, Zhang ShD (2008) Gaseous product generated by radiation degradation of N, N-diethylhydroxydrolamine aqueous solution. Nucl Sci Tech 19:79–82
Chen ZhH, Wu YB (2004) Determination of ammonium by ion chromatography for spectrophotometry. Mod Sci Instrum 5:64–65 (in Chinese)
Wu GP, Wei FSh (1992) Determination of nitrite in the wet precipitation—N-(1-naphthyl)-1,2-diaminoethane dihydrochloride spectrophotometry. GB-13580.7-92:1-2 (in Chinese)
Ha HF, Wu JL (2002) Radiation chemistry of polymer—principle and application. Peking University Press, Beijing
Kuruc J, Sahoo MK, Kuran P (1993) Radiation chemical yields of main volatile products in the gamma-radiolysis of nitrobenzene and isomeric dinitrobenzene, nitrophenol, and nitroaniline solutions in carbon tetrachloride. J Radioanal Nucl Chem 174(1):103–114
Krishnamurthy MV, Sipahimalani AT (1995) Radiolytic degradation of TBP-HNO3 system: gas chromatographic determination of radiation chemical yields of n-Butanol and nitrobutane. J Radioanal Nucl Chem Lett 199:197–206
Li HB, Su Zh, Cong HF, Song FL, Wang XR, He H, Liu ZhY, Lin CSh (2012) α and γ irradiation stability of 30% TBP-kerosene-HNO3 systems. J Nucl Radiochem 34:281–285 (in Chinese)
Karraker DG (2002) Radiation chemistry of acetohydroxamic acid in the Urex process (WSRC-TR-2002-00283), U.S. Department of Commerce, National Technical Information Service: Springfield, 1–8
Burton M (1951) An introduction of radiation chemistry. J Chem Educ 28:404–420
Spinks JWT, Woods RJ (1976) An introduction to radiation chemistry, 2nd edn. New York, Wiley
Samuni Y, Samuni U, Goldstein S (1820) The mechanism underly nitroxyl and nitric oxide formation from hydroxamic acid. Biochim Biophys. Acta 10:1560–1566
Samuni A, Goldstein S (2011) One electron oxidation of hydroxamic acid: The intermediacy of nitroxyl and peroxynitrite. J Phys Chem A 115:3022–3302
Xu YP, Mull CD, Bonifant CL, Yasaki G, Palmer EC, Shields H, Ballas SK, Kim-Shapiro DB, King SB (1998) Nitrosylation of sickle cell hemoglobin by hydroxyurea. J Org Chem 63:6452–6453
King S (2003) The nitric oxide producing reactions of hydroxyurea. Curr Med Chem 10:437–452
Gutiérrez MM, Almaraz AE, Bari SE, Amorebieta JAOV (2015) The HNO donor ability of hydroxamic acids upon oxidation with cyanoferrates(III). J Coord Chem 17–18:3236–3246
Lefort M, Tarrago X (1961) Significance and function of NH2 radical in the decomposition of hydroxylamine in aqueous solution by ionizing radiation. J Inorg Nucl Chem 16(3–4):169–189 (in French)
Shirota FN, DeMaster EG, Lee MJC, Nagasawa HT (1999) Generation of nitric oxide and possibly nitroxyl by nitrosation of sulfohydroxamic acids and hydroxamic acids. Nitric Oxide-Biol Ch 3(6):445–453
Bonner FT, Hughes MN (1988) The aqueous solution chemistry of nitrogen in low positive oxidation states. Comments Inorg Chem 7:215–223
Horne GP, Gregson CR, Sims HE, Orr RM, Taylor RJ, Pimblott SM (2017) Plutonium and americium alpha radiolysis of nitric acid solutions. J Phys Chem B 121:883–889
Horne GP, Grimes TS, Bauer WF, Dares CJ, Pimblott SM, Mezyk SP, Mincher BJ (2019) Effect of ionizing radiation on the redox chemistry of penta-and hexavalent americium. Inorg Chem 58:8551–8559
Mincher BJ, Precek M, Mezyk SP, Elias G, Martin LR, Paulenova A (2013) The redox chemistry of neptunium in γ-irradiated aqueous nitric acid. Radiochim Acta 101:259–266
Bhattacharyya PK, Veeraraghavan R (1977) Reaction between nitrous acid and hydrogen peroxide in perchloric acid medium. Int J Chem Kinet 9(4):629–640
Anbar M, Taube H (1954) Interaction of nitrous acid with hydrogen peroxide and with water. J Am Chem Soc 76(24):6243–6247
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Wang, S.Y., Wang, J.H. et al. γ-Ray radiolysis of dihydroxyurea in water and its radiolytic by-products. J Radioanal Nucl Chem 333, 1137–1145 (2024). https://doi.org/10.1007/s10967-024-09375-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09375-z