Abstract
Mineralogical study showed that the Yuejin uranium ore is from a low grade, high carbonate and high pyrite sandstone type uranium deposit. Uranium leaching rate, uranium speciation of leachate, leaching kinetics and precipitation analysis were investigated under the conditions of different acidity, liquid–solid ratio and H2O2 concentration. The test results showed that the weak acid leaching process can be used to develop high carbonate type uranium deposits, however, because of the high content of pyrite, other oxidants except high concentration H2O2 need to be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of nuclear power around the world, uranium resources have become particularly imperative [1, 2]. In recent years, low-grade uranium resources have attracted increasing attention with the continuous depletion of middle and high-grade uranium reserves [3,4,5]. In the past 30 years, in situ leach (ISL) has become the main method for mining medium and low-grade sandstone-type uranium deposits [6]. Traditionally, in situ leach (ISL) can be divided into acidic leaching and alkaline leaching according to the different PH values of the leaching solution [7, 8]. Generally, sulfuric acid/iron sulfate and carbonate/bicarbonate are common leachants in acidic and alkaline leaching processes, respectively. Acidic leaching process possesses high leaching intensity and short leaching period while being harmful to the environment and easy to produce CaSO4 blocking formation. Alkaline leaching process exhibits low leaching intensity, long leaching cycle, and little harm to the environment. However, it is easy to produce CaCO3 blocking formation. It has been reported that formation blockage is one of the key factors restricting ISL industrial applications [9, 10].
Neutral leaching technology, evolving from acidic leaching technology and alkaline leaching technology, is a promising new green technology. Compared with traditional leaching technology, neutral uranium leaching technology presents small damage to the environment and is difficult to produce CaSO4 and CaCO3 precipitation formation blockage while maintaining high leaching intensity. Thus, it is extremely suitable for uranium deposits with high carbonate contents. Currently, the neutral leaching process is majorly composed of CO2 + O2 neutral leaching and weak acid neutral leaching [11]. Essentially, it allows HCO3− to complex with uranium to form uranyl ions while the means of obtaining HCO3− are different. HCO3− is produced by the reaction of CO2 and low concentration H2SO4 with calcite or dolomite in ore, respectively [12, 13]. Many industrial applications of the CO2 + O2 neutral leaching process have been reported, such as Highland uranium deposit in the USA, Mengqgur uranium deposit in China, and ELZPA uranium deposit in Pakistan [14,15,16,17]. However, there is little research on the weak acid neutral leaching process, especially on the mechanism of the process. Therefore, the weak acid leaching process should be further explored.
The mineralogical characteristics of uranium ore will affect the selection of different leaching processes [18,19,20,21,22]. Process mineralogy research guides the smelting of ore minerals based on physicochemical properties such as mineral composition, geochemical composition, structural structure, and occurrence forms of elements [23,24,25,26]. Yuejin sandstone-type uranium deposit is located in the HuaTuGou region on the northwest edge of the Qaidam Basin. The uranium-bearing rock series is Shizigou Formation, which is characterized by high carbonate content. The predecessors mainly studied its sedimentary characteristics, stratigraphic sequence framework, formation mechanism, and source characteristics, as well as its relationship with uranium ore. However, its leaching technology remains unclear [27].
In this paper, the mineralogical characteristics of the Yuejin uranium ore were studied with an optical microscope, SEM, XRD, XRF, EPMA, and ICP to demonstrate the uranium leaching effect of the weak acid neutral leaching process on high carbonate uranium deposits. The effects of initial acidity, liquid–solid ratio, initial H2O2 concentration, reaction time, and hydrochemical conditions on uranium leaching were explored through static batch tests. Besides, a shrinking core model was employed to reveal the kinetic characteristics of weak acid uranium leaching. The presence of uranium species in leach solution was calculated by using PHRECQCI geochemical model software. Then, the risk of formation plugging was evaluated by CaCO3 saturation index.
Materials and methods
Preparation of uranium ore
As shown in Fig. 1, uranium ores were taken from several cores of six boreholes of the Yuejin uranium deposit in Qaidam Basin, Qinghai Province, China. The ore from each hole was crushed and mixed according to the natural grain size, and then sieved according to the six grain classes of > 5, 5 ~ 2.5, 2.5 ~ 1, 1 ~ 0.5, 0.5 ~ 0.2, and < 0.2 mm. It was then mixed according to the proportion of each grain class. A representative sample was prepared by coning and quartering for chemical analysis.
Mineralogical study methods
The rock analysis was entrusted to ALS Chemex (Guangzhou, China) Co., Ltd., which included analysis of major elements, ferrous iron, total sulfur, sulfate sulfur, sulfide sulfur, inorganic carbon (carbonate), organic carbon, uranium, and other trace elements.
Polarized light microscopy analysis was conducted by Petrochina Qinghai Oilfiled Company, and authenticated by the Testing Research Institute of Geology Party No. 216, CNNC, using the ZEISS Axio Imager. The analysis method was performed in accordance with the national standard (GB/T 17412.1.2.3-1998).
The X-ray diffraction analysis was performed at the State Key Laboratory of Nuclear Resources and Environment, ECUT, with the Bruker D8-A25 manufactured by Bruker, Germany. Phase identification was achieved using JADE version 9.3 in combination with information from the ICSD and ICDD diffraction databases [28].
The microscopic observation and microanalysis were conducted at the State Key Laboratory of Nuclear Resources and Environment, ECUT, and the back-scattering images were observed through an FEI Nova NanoSEM 450 field emission scanning electron microscope. Elemental testing was conducted using an Oxford X-Max 20 EDS, and the elemental contents of minerals were analyzed using a JEOL JXA-8530F Plus FE-EPMA. The test was performed according to the national standard (GB/T 15617-2002) [29].
Leaching methods
To investigate the applicability of the weak acid leaching process to uranium ore from the Yuejin deposit, three batches of tests were designed to leach uranium ore by static leaching in a sealed beaker with differently prepared leaching agents. The sampling times were set as 1, 3, 7, 13, 25, 49, 97, 145, and 193 h.
In the first batch of tests, weak acid acidity conditions were tested, with five acidity levels of 0, 0.15, 0.3, 0.5, and 1 g L−1. The amount of ore used was 100 g, and the amount of lysis leaching solution was 500 mL.
In the second batch of tests, the liquid–solid ratio was tested. Here, the best weak acid acidity found according to the results of weak acid acidity condition testing was selected. Four liquid–solid ratios were tested, namely 80 mL:100 g, 170 mL:100 g, 250 mL:100 g, and 500 mL:100 g.
In the third batch of tests, the H2O2 concentration conditions were tested. Here, the weak acid acidity and liquid–solid ratio were selected based on the results from the first two batches of tests, and four H2O2 (30%) concentrations 0, 1, 2, and 3 g L−1 were tested.
The alkalinity of leachate was titrated according to the GB/T8538-2008 (4.10 and 4.42) standard solution of hydrochloric acid-phenolphthalein methyl orange, and the generated HCO3− was calculated according to the volume of acid consumption. The pH and Eh of the leachate were determined using a Mettler-Toledo FE28 precision acidity meter. The concentrations of uranium ion and other metal ions were determined by ICP-OES (Agilent 5100).
Uranium species analysis software
PHREEQCI is the most widely used hydrogeochemical simulation software in the world because of its friendly interface, powerful function and wide practicability. The software, officially launched in 1995, was written by the US Geological Survey. Based on the PHREEQCI program to calculate the balanced distribution of uranium leachate.
Leaching kinetic model
To determine the relationship between the amount of leached uranium and leaching parameters during the leaching process, a kinetic analysis of the leaching process was performed. The static leaching test is a liquid–solid multiphase reaction, where the reaction occurs at the interface and the interfacial reaction rate depends on the interfacial concentration of reactants as well as the interfacial concentration of products, while the overall reaction rate depends on the slowest interfacial reaction rate among them [30,31,32,33]. Therefore, the shrinking core models (MSL) can be used to describe the reaction, and the three-dimensional phase interface reaction equation and the three-dimensional diffusion equation were chosen for linear fitting, as follows:
where kd is the diffusion rate constant, kc is the interfacial reaction rate constant, F is the uranium leaching rate, and t is the reaction time.
Results and discussion
Mineralogical study of leaching process
As shown in Fig. 2a, b, the color of the uranium ore from the Yuejin deposit is greenish gray and massive structure and lithology of argillaceous siltstone, with relatively loose consolidation and blistering when encountering hydrochloric acid. As shown in Fig. 1c, the overall particle size of the uranium ore from the Yuejin deposit is small and poorly sorted, with 71.03% of the mass of particles being ≤ 1 mm in size, indicating that the ore has a high specific surface area and, therefore, there is a greater probability of liquid–solid phase mutual contact [34], though it may also have poor permeability.
As shown in Fig. 3a–d, the uranium ore was microscopically observed that the detrital minerals mainly included quartz, plagioclase, potassium feldspar, and limestone debris. The main components of the cement were sericite microcrystals and putty crystal calcite, and other metallic minerals were mainly pyrite and magnetite.
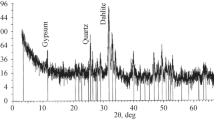
The XRD pattern is shown in Fig. 4, and the semi-quantitative analysis of minerals is shown in Table 1. The pH of the neutral leaching solution was mainly maintained at 6.0–7.0, and the feldspar minerals, such as quartz and feldspar, did not react under these pH conditions [35, 36]. Kaolinite, as a clay mineral, has a large specific surface area, and its presence may adsorb some of the dissolved U(VI) [37, 38]. Calcite and dolomite, as carbonate minerals, play a major role in the weak acid leaching process by reacting with H2SO4 to produce large amounts of HCO3−. During the weak acid leaching process, Pyrite and magnetite not only produce iron precipitates that block formation but also compete with tetravalent uranium minerals to consume oxidants in the leaching solution [39].
The main chemical components of the ore are shown in Table 2. The inorganic carbon (CO2) contents of the ore were 3.8%, indicating a high carbonate content in the ore. The U content in the ore was 139 ppm, of which U(IV) accounted for 63.6% and U(VI) accounted for 36.4%, indicating that the ore is of low uranium grade and mainly dominated by tetravalent uranium minerals. The solubility of tetravalent uranium oxide (UO2) was very low, only 5 × 10−8 g L−1 [40], and only it entered the water as uranyl ion and its complexes following oxidation into hexavalent uranium.
The backscatter micrographs of uranium ore are shown in Fig. 5, and the composition as determined from EPMA analysis is shown in Table 3. The uranium minerals in the uranium ore of the Yuejin deposit were mainly brannerite, pitchblende, and coffinite. The Ti element content of 0.255% in the Yuejin deposit ore indicates that a significant portion of the uranium minerals in the ore may be brannerite, which is more difficult to leach compared to other uranium minerals [41]. The minerals were often wrapped in pyrite and surrounded by calcite. The symbiotic relationship between uranium minerals and pyrite is obvious. In the leaching process, pyrite not only consumes oxidants competitively with uranium minerals but also releases H+ during the oxidation process, which destroys the local alkaline environment and affects the complexation between carbonate and uranyl ions. The released SO42− together with Ca2+ released by the dissolution of carbonate minerals will result in the formation of gypsum precipitation, which blocks the holes [42, 43].
The effect of acidity on uranium leaching
Uranium leaching results
As shown in Fig. 6, the terminal weak acid leaching rates for at 0, 0.15, 0.3, 0.5, and 1 g L−1 H2SO4 were 9.61%, 13.18%, 13.26%, 18.77%, and 19.90%, respectively. The terminal uranium leaching rates for all acidity tests were relatively low, and for the highest acidity (1 g L−1 H2SO4), the leaching rate of 19.90% was far below the percentage (36.4%) of hexavalent uranium in the ore, indicating that most of the hexavalent uranium in the ore was not leached out (it should be noted that no oxidant was added in this test). The overall positive correlation between the uranium leaching rate and the acidity was not significant in the local interval.
Hydrochemical characterization
The presence of uranium in solution as a complex is controlled by two main conditions: the complexation chemistry of uranium and the chemical conditions of the aqueous medium [44].
As shown in Fig. 7a, when the weak acid solution with 0 g L−1 H2SO4 came into contact with the ore, some carbonate minerals were dissolved into the solution, resulting in an instantaneous increase in the solution pH from 6.97 to 8.08. With the ionization equilibrium of carbonic acid and its derivatives, the pH stabilized to about 7.39 after 50 h. When the weak acid solutions with 0.15, 0.3, 0.5, and 1 g L−1 H2SO4 solutions were in contact with the ore, the carbonate minerals in the ore reacted rapidly with the H2SO4 in the solution, resulting in an instantaneous increase in the solution pH from about 2 to 6.19, 5.54, 5.29, and 3.57, respectively, and as the reaction continued, the solution pH increased continuously and rose to 7.39, 7.33, 7.20, and 6.67, respectively, up to 193 h.
In weak acid leaching, the initial sulfuric acid concentration in the solution determines the total concentration of carbonic acid derivatives produced after sufficient reaction with the carbonate minerals, as shown in Fig. 8. The pH determines the distribution coefficient of each carbonic acid derivative in the solution [45].
As shown in Fig. 7a,b, the HCO3− in solution during the weak acid leaching test with 0 g L−1 H2SO4 was mainly from the dissolution of a small amount of carbonate minerals in the ore, and the HCO3− concentration stabilized at 61.22 mg L−1 after reaching the maximum at 50 h. The HCO3− concentration curves of the weak acid leaching tests at 0.15, 0.3, and 0.5 g L−1 H2SO4 were essentially the same up to 25 h. As the reaction proceeded beyond this point, the pH of the latter two solutions gradually increased, and the final HCO3− concentration of the curves were 82.64, 122.44, and 168.36 mg L−1, respectively. As the reaction proceeded, the pH of the latter two solutions gradually increased, and the HCO3− concentration curves gradually diverged. The final HCO3− concentrations of the three curves were 82.64, 122.44, and 168.36 mg L−1, respectively, while the pH during the weak acid leaching test at 1 g L−1 H2SO4 was less than 5.69 before 100 h, and the carbonic acid derivatives in the solution mainly existed in the form of H2CO3. As the reaction proceeded, the pH gradually increased, and the HCO3− concentration also gradually increased, and the final HCO3− concentration was 153.05 mg L−1.
In neutral leaching systems, uranium exists mainly as uranyl carbonate, and the activity of carbonic acid and its derivative ions is an important condition that affects the solubility of uranium complexes [46]. As shown in Fig. 7b, c, the uranium leaching concentration and rate were strongly correlated with the bicarbonate concentration in the solution, except for in the case of 1 g L−1 H2SO4 weak acid leaching.
In summary, 0.5 g L−1 H2SO4 acidity was chosen for subsequent testing of liquid–solid ratio conditions.
Uranium speciation
Uranyl ions are stable only within a certain pH range, beyond which conversion occurs [47]. According to the pH and chemical components of the leach solution, the presence of uranium in water was calculated using the geochemical model software PHREEQCI for weak acid leaching with 0.5 g L−1 and 1 g L−1 H2SO4. As shown in Fig. 9a, the uranium was mainly present as UO2CO3 at the beginning of the weak acid leaching test with 0.5 g L−1 H2SO4, and as the pH and HCO3− concentration in the solution increased, and the UO2CO3 was gradually converted to UO2(CO3)22− and UO2(CO3)34−. UO2(CO3)22− was present around the halfway point of the test and was gradually converted to UO2(CO3)34− toward the end. As shown in Fig. 9b, uranium was mainly present as UO2SO4 and UO22+ at the beginning of the weak acid test for 1 g L−1 H2SO4, and UO2SO4 and UO22+ were gradually converted to UO2CO3 as the pH and HCO3− concentration in the solution increased. With the sudden increase in HCO3− concentration in the solution at 100 h, the uranium in the solution was converted to UO2(CO3)22−. It should be noted that UO22+ in weak acid leaching test at 1 g L−1 H2SO4 was gradually converted to UO2(OH)2 as the pH increased, and hydrolytic precipitation may occur when the pH exceeds 4.6.
The effect of liquid–solid ratio on uranium leaching
Uranium leaching results
As shown in Fig. 10, the weak acid leaching rates for different liquid–solid ratios of 80, 170, 250, and 500 mL were 20.6%, 22.47%, 26.21%, and 18.77%, respectively. The terminal uranium leaching rate was lower for all liquid–solid ratio condition tests, with weak acid (250 mL) > weak acid (170 mL) > weak acid (80 mL) > weak acid (500 mL).
Hydrochemical characterization
As shown in Fig. 11a, the solution pH was obviously affected by the liquid–solid ratio, which showed that the higher the liquid–solid ratio, the more residual acid that remained in the solution after the reaction between the sulfuric acid and the carbonate minerals within the ore, and the lower the solution pH. As shown in Fig. 11b, the change curves of the HCO3− concentrations in the solutions of weak acid for 80, 170, and 250 mL were almost synchronized, while the HCO3− concentration for 500 mL weak acid was much lower than the other groups because the solution pH remained below 6.5 for a long time, meaning that the carbonate derivatives existed mainly in the form of H2CO3. As shown in Fig. 11c, the uranium leaching rate value for weak acid at 250 mL was much higher than for the other groups. In terms of the change trend, the uranium leaching rate increased rapidly before 25 h, at which point it started slowing down. The uranium leaching rate value for 170 mL weak acid was slightly higher than for 80 mL. In terms of the change trend, both were nearly completely synchronized. The uranium leaching rate value was much lower for 500 mL than the other groups and, in terms of the change trend, exhibited a steady increase.
In summary, the acidity of 0.5 g L−1 H2SO4 and the liquid–solid ratio of 250 mL:100 g was chosen for the subsequent H2O2 concentration condition testing.
Leaching kinetic mechanism
The kinetic fitting results are shown in Fig. 12 and Table 4. The reaction rates of weak acid for 80, 170, and 250 mL were mainly controlled by the diffusion of the product layer, indicating that the higher the liquid–solid ratio, the faster the reaction rate; weak acid (500 mL) was mainly controlled by the chemical reaction, indicating that the HCO3− concentration in the solution was too small, thus limiting the uranium leaching rate. This is consistent with the experimental results.
Precipitation analysis of leaching process
The dissolution and precipitation of calcium carbonate is controlled by its solubility product, and the solubility product of calcium carbonate is influence.
d by the concentrations of Ca2+ and HCO3− in water as well as its pH, which governed each other through inverse correlation.
where SI is the calcium carbonate saturation index, SI > 0, calcium carbonate supersaturation, precipitation, and vice versa dissolution, and KSP is the calcium carbonate dissolliquidution equilibrium constant.
The relationship between calcium carbonate SI and time is shown in Fig. 13. The higher the -solid ratio of weak acid (0.5 g L−1 H2SO4), the lower the SI. The later the time to reach zero, the longer the contact time and the higher the SI, indicating that in situ weak acid leaching should not last for too long in terms of the contact time between the leaching solution and formation because of the risk of plugging the formation via calcium carbonate precipitation.
The effect of H2O2 concentration on uranium leaching
Uranium leaching results
As shown in Fig. 14, the terminal weak acid leaching rates for 0, 1, 2, and 3 g L-1 H2O2) were 26.20%, 21.91%, 23.97%, and 15.99%, respectively, which indicates that the leaching rates after the addition of H2O2 were all lower than those without the addition of H2O2. The trend shows that the higher the of H2O2 concentration, the lower the leaching rate.
As shown in Fig. 15, the uranium leaching rate curve of for 0 g L−1 H2O2 showed a stable increasing trend, while the uranium leaching rates for 1, 2, and 3 g L−1 H2O2 showed irregular phenomena of alternating rising and falling, and it is speculated that uranium precipitation occurred during the leaching process.
H2O2 and uranium precipitation analysis
To investigate the mechanism of this phenomenon, a set of exploratory experiments was designed, in which 0.04 mL of H2O2 (30%) was added dropwise to 100 mL of neutral leachate with a uranium concentration of 24.13 mg L−1, and the residual uranium concentration was monitored at different times.
As shown in Fig. 16, after the dropwise addition of H2O2, the residual uranium concentration gradually decreased and stabilized at 9.86 mg L−1 after 10 h, indicating that during the neutral leaching process with H2O2 as the oxidizing agent, the addition of H2O2 led to precipitation of the leached uranium while simultaneously oxidizing the tetravalent uranium mineral. In the uranium hydrometallurgical process, hydrogen peroxide can be used as a precipitant for uranium, which precipitates uranium at pH = 3.5 through the following reaction [48,49,50].
Since the generated uranium peroxide was insoluble and the solution pH was around 7 during the weak acid neutral leaching process, the of H2O2 concentration must be controlled to avoid uranium precipitation due to excess H2O2.
Conclusions
Through a mineralogical study of the leaching process and a batch static leaching test, we evaluated the difficulty of leaching uranium ore from Yuejin deposit and explored the applicability of a weak acid neutral leaching process for this purpose. The uranium ore is small in grain size and rich in carbonate minerals and pyrite. The main uranium minerals are brannerite, pitchblende, and coffinite. Pyrite and calcite are commonly found around uranium minerals with high tetravalent uranium content, and, overall, the leaching of uranium ore was difficult. The weak acid leaching process is able to satisfactorily complex hexavalent uranium through the HCO3− generated by sulfuric acid in the leaching solution with carbonate minerals. When the pH of the solution is maintained around 7, uranium exists mainly in the forms of UO2(CO3)22− and UO2(CO3)34−. The calculation of the leachate CaCO3 saturation index showed that the saturation index value of weak acid leaching becomes higher with a longer contact time, lower with a higher liquid–solid ratio, and lags behind in reaching the zero point, indicating that the risk of CaCO3 blockage still exists in the weak acid leaching process. High concentrations of H2O2, while oxidizing the tetravalent uranium minerals, also lead to precipitation of the uranium already present in solution, negatively affecting leaching, which is dependent on the uranium being in solution. However, it was found that the presence of reducing minerals, such as pyrite, which is present at high levels in the uranium ore, limits the need to add high concentrations of oxidant to the solution. Hence, future research will focus on the selection of a suitable oxidant to improve the effectiveness of uranium leaching.
References
Schneider M, Froggatt A (2012) 2011–2012 world nuclear industry status report. B Atom Sci 68(5):8–22
Sarkar A (2019) Nuclear power and uranium mining: current global perspectives and emerging public health risks. J Public Health Policy 40(4):383–392
Thakur S, Chudasama B, Porwal A (2018) Global grade-and-tonnage modeling of uranium deposits. IAEA TECDOC SERIES 218
Anjum F, Shahid M, Akcil A (2012) Biohydrometallurgy techniques of low grade ores: A review on black shale. Hydrometallurgy 117:1–12
Mudd GM (2014) The future of Yellowcake: A global assessment of uranium resources and mining. Sci Total Environ 472:590–607
IAEA, Vienna, IAEA TECDOC 1629 (2009) World Distribution of Uranium Deposits (UDEPO) with Uranium Deposit Classification; International Atomic Energy Agency: Vienna, Austria
Sreenivas T, Rao KA, Rao MM et al (2010) Autoclave leaching of refractory uranium minerals. In: Proceedings of the XI International Seminar on Mineral Processing Technology (MPT-2010). Vol. 1. No. Section 8. Allied Publishers, New Delhi
Gilligan R, Nikoloski AN (2015) The extraction of uranium from brannerite: A literature review. Miner Eng 71:34–48
Zhanxue S, Wenjie M, Yajie L et al (2021) Research progress on groundwater contamination and remediation in In-situ leaching uranium mining. Front Earth Sci Prc. https://doi.org/10.13745/j.esf.sf.2021.2.11
Xiaoyu Y, Chen G, Yi Z (2020) Analysis of the relationship between CO2 + O2 ground leaching over omitted out uranium concentration and HCO3- concentration in sandstone type uranium ore. Uranium Min Metall 39(01):11–16
Qinglin Z, Yipeng Z, Zhijun M, et al (2020) Field trial on enhanced in-situ leaching of Uranium by CO2 + O2 leaching. Non-Ferr Met 12:48–53
Haibo W, Ben N, Jing Y, et al (2015) Impact factors of a sandstone uranium mine in Inner Mongolia. In: Proceedings of the 2015 Academic Annual Conference of the Chinese Nuclear Society. Vol 1:505–509. Chinese Nuclear Society
Yuan Y, Lei Z, Guoping J, et al (2015) Evaluation of neutral ground dip uranium mining conditions in a sandstone uranium deposit. In: Proceedings of the 2015 Academic Annual Conference of China Nuclear Society. Vol 1:531–535. Chinese Nuclear Society
Pingru Z, Mcgonagle L (2004) Alkaline ground leaching process for uranium mining at Highland uranium mine USA (continued). Metall Uranium Min. https://doi.org/10.13426/j.cnki.yky.2004.03.004
Yong Z, Qunying H, Yipeng Z (2016) Experiments on uranium mining by weak acid neutral ground leaching in high carbonate type uranium deposits. Nonferrous Met 2:33–37
Yong Z, Yipeng Z, Qinglin Z (2014) Study on the saturation state of calcium carbonate of uranium mined by weak acidic ground leaching in the Mengqiqur deposit. Nonferrous Met 12:24–27
Asghar F, Sun Z, Chen G et al (2020) Geochemical characteristics and uranium neutral leaching through a CO2 + O2 system-an example from uranium ore of the ELZPA ore deposit in Pakistan. Metals 10(12):1616
Huaming L, Ling L, Yunbiao P et al (2021) Study on the relationship between clay minerals and oxide zone genesis of sandstone uranium deposits in the northern Ordos Basin. Chin J Geol 56(01):303–320
Qiang Z, Rengan Y, Guangyao L et al (2021) Associated mineral assemblage of sandstone type uranium ore in northeastern Ordos Basin and its geological significance. Geotecton Metallog 45(02):327–344
Ran Z, Lijuan Y, Feipeng D et al (2021) Application of automatic mineral analysis technology to identify minerals and occurrences of elements in sandstone-type uranium deposits in the ordos basin. Rock Miner Anal 40(01):61–73
Kai Z, Guangrong L, Yipeng Z et al (2019) Research progress of leaching of sandstone type uranium ore. Non Ferr Met 6:40–48
Guangrong L, Yipeng Z, Kai Z, et al (2021) Researh progress on mineral leaching technology of sandstone type uranium deposits. Nonferrous Met. DOI:CNKI:SUN:METE.0.2021-08-003.
Mingsheng P, Xiaoweng L, Yu L et al (2012) The main advances of process mineralogy in china in the last decade. Bull Min Petro Geochem 31(3):210–217
Xiao Y, Fang M, Fu Q, et al (2018) New techniques and concepts in process mineralogy. Conserv Util Min Resour. https://doi.org/10.13779/j.cnki.issn1001-0076.2018.03.009
Lijuan T, Guangwei Z (2014) Application of process mineralogy in Benefication. Mod Min 30(12):68–71
Chunfeng B, Tengfei L, Gali Z (2021) Process mineralogy study of one lead-zinc ore in inner mongolia and characteristics analysis of its flotation product. Yunan Metallu 50(04):19–24
Chao Z, Qinghong S, Rengan Y, et al (2020) Analysis of the relationship between sedimentary characteristics and uranium deposits from the Neogene Shizigou Formation in Huatugou area Northwest Qaidam Basin. Geol. China. https://kns.cnki.net/kcms/detail/11.1167.P .20201010.1111.005.html
Andong W, Jianjun W, Lei L (2015) SEM-EDS and XRD studies of the Huoqiu Group BIF at the southeast margin of North China and its geological significance. J East China Univ Sci Technol 38(01):23–31
Jianjun W, Chunrong P, Jie Y et al (2021) Application of electron probe-Scanning electron microscopy to study the characteristics of rare earth minerals in the uranium rare polymetallic deposit in Huayangchuan Shaanxi. Rock Miner Test 40(01):145–155
Linlin X, Hongying Y, Yipeng Z (2020) Experimental study on leaching uranium ore with five low molecular weight organic acids. J Mater Metall 19(03):203–208
Ubaldini S (2021) Leaching kinetics of valuable metals. Metals 11(1):173
Zakia SA, Rashad MM, Mohamed SA et al (2020) Kinetics of uranium leaching process using sulfuric acid for Wadi Nasib ore South western Sinai Egypt. Aswan Univ J Environ Stud 1(2):171–182
Mahmoud KF, Kamal HM, Shehata MR et al (2020) Leaching kinetics of uranium from Playa deposits at Atmur El-Kibeish area south western desert Egypt. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1849650
Xuegang W, Zhanxue S, Yajie L et al (2019) Effect of particle size on uranium bioleaching in column reactors from a low-grade uranium ore. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.02.065
Kang P (2017) Analysis of the characteristics of sandstone type uranium ore and its mining method. Groundwater 39(03):226–229
YiPeng Z, Zhaoli S, Weijun S (2015) Exploration on the classification method of uranium mining process by ground leaching. Non-Ferr. Met 01:37-41
Kang Z, Chong C, Jianying S (2018) Effect of humic acid-kaolinite colloids on the migration process of uranium(VI) in saturated porous media. J Soil Water Conserv 32(06):286–293
Zuoyong D, Yangchun L, Chunchun W (2019) Adsorption of U(VI) by ilmenite and kaolinite. Nucl Chem Radiochem 41(05):503–508
Hao L, Kaigang H, Jinfang M (2021) Experimental study on integrated leaching of uranium and pyrite from uranium ores containing sulfur-carbonate. Nonferrous Met Eng 11(01):56–61
Langmuir D (1978) Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim Cosmochim Acta 42(6):547–569. https://doi.org/10.1016/0016-7037(78)90001-7
Xingbo Z, Zhanxue S, Baoqun H (2014) Changes of uranium minerals during bioleaching of 745 ore. Nonferrous Met 4:38–41
Wenrui J, Zhihong T, Shu Z (2021) Progress of research on the mechanism and kinetic influencing factors of surface oxidation of pyrite. Min Met. https://doi.org/10.19614/j.cnki.jsks.202103013
Wuyang H, Kaixuan T, Yongmei L (2021) Groundwater chemistry and sulfur isotope characteristics of a CO2 + O2 ground leach uranium mining area in Xinjiang. Res Dev Min. https://doi.org/10.13827/j.cnki.kyyk.2021.01.020
Linlin X, Hongying Y, Yipeng Z et al (2020) Neutral leaching performance test of ore from a sandstone uranium deposit. Nonferrous Met 3:38–44
Hongxiao T (1978) Calculation of carbonate balance and pH adjustment (above). Environ Sci. https://doi.org/10.13227/j.hjkx.1979.05.006
Yipeng Z, Lingling X, Guangrong L (2018) Characteristics of oxidant-free alkaline leaching of sandstone type uranium ores. Nonferrous Met 12:37–42
Hunter E (2013) On the leaching behavior of uranium-bearing resources in carbonate-bicarbonate solution by gaseous oxidants. Colorado School of Mines
Xizhen G, Sirong L, Erhua G et al (1997) Precipitation of uranium from the leach solution of uranium ore from Haematang with hydrogen peroxide. Metall Uranium Min. https://doi.org/10.13426/j.cnki.yky.2002.02.006
Pingru Z, Yijun Z, Jianhua L (2002) A new uranium recovery process:two-step precipitation by iron alum-hydrogen peroxide. Uranium Min Metall. https://doi.org/10.13426/j.cnki.yky.2002.02.006
Lei Z, Yuan Y, Guoping J, et al (2015) Transfer and precipitation of uranium and calcium in a uranium mine. In: Proceedings of the 2015 Academic Annual Conference of the Chinese Nuclear Society. Vol 1:410–414. Chinese Nuclear Society
Acknowledgements
This research was supported by the National Natural Science Foundation of China (U1967209), the Natural Science Foundation of Jiangxi Province of China (20202BABL203033) and the Petrochina Qinghai Oilfiled Company research projects, “Laboratory technological conditions test on in-situ leaching of Uranium and associated elements in Yuejin sandstone uranium deposit in southwest Qaidam Basin”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, S., Wei, X., Liu, J. et al. Weak acid leaching of uranium ore from a high carbonate uranium deposit. J Radioanal Nucl Chem 331, 2583–2596 (2022). https://doi.org/10.1007/s10967-022-08323-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08323-z