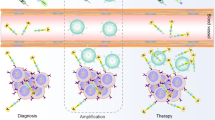
Abstract
Amplification pretargeting strategies have great potential in nuclear medicine to increase the tumoral radioactivity concentration compared to conventional pretargeting. In this work, the dendrimer polyamidoamine generation 4 (PAMAM G4) was conjugated to multiple copies of peptide nucleic acid (PNA) as a signal amplification platform, which could combine with the antibody of CC49-cPNA and the tracer of 18F labeled complementary peptide nucleic acid (18F-cPNA) in biodistribution experiments to determine the signal amplification effect in vivo. The mice in the Amplification Group exhibited expected tumoral uptake (3.21 ± 0.77%ID/g), more than double that in the Pretargeting Group (1.21 ± 0.03%ID/g). Therefore, this work confirmed the signal amplification effect of dendrimer-PNA in vivo.








Similar content being viewed by others
References
Goodwin DA et al (1997) Pretargeting - General principles. Cancer-Am Cancer Soc 80:2675–2680
Goodwin DA et al (1988) Pre-Targeted Immunoscintigraphy of Murine Tumors with Indium-111-Labeled Bifunctional Haptens. J Nucl Med 29:226–234
Hnatowich DJ et al (1987) Investigations of avidin and biotin for imaging applications. J Nucl Med 28:1294–1302
Dou SP et al (2014) Differentiation between temporary and real non-clearability of biotinylated IgG antibody by avidin in mice.Front Pharmacol.5
Reardan DT et al (1985) Antibodies against metal chelates. Nature 316:265–268
Kuijpers WHA et al (1993) Specific Recognition of Antibody Oligonucleotide Conjugates by Radiolabeled Antisense Nucleotides - a Novel-Approach for 2-Step Radioimmunotherapy of Cancer. Bioconjug Chem 4:94–102
Chang CH et al (2002) Molecular advances in pretargeting radioimunotherapy with bispecific antibodies. Mol Cancer Ther 1:553–563
Bos ES et al (1994) In-Vitro Evaluation of DNA-DNA Hybridization as a 2-Step Approach in Radioimmunotherapy of Cancer. Cancer Res 54:3479–3486
Agard NJ et al (2005) A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems (vol 126, pg 15046, 2004). J Am Chem Soc 127:11196–11196
Rossin R et al (2010) In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew Chem Int Edit 49:3375–3378
Chen X et al (2008) Synthesis and in vitro characterization of a dendrimer-MORF conjugate for amplification pretargeting. Bioconjug Chem 19:1518–1525
Wang Y et al (2001) Pretargeting with amplification using polymeric peptide nucleic acid. Bioconjug Chem 12:807–816
Agrawal S et al (1995) Pharmacokinetics of antisense oligonucleotides. Clin Pharmacokinet 28:7–16
Karkare S et al (2006) Promising nucleic acid analogs and mimics: characteristic features and applications of PNA, LNA, and morpholino. Appl Microbiol Biot 71:575–586
Smolina IV et al (2003) Sequence-universal recognition of duplex DNA by oligonucleotides via pseudocomplementarity and helix invasion. Chem Biol 10:591–595
Briones C et al (2012) Applications of peptide nucleic acids (PNAs) and locked nucleic acids (LNAs) in biosensor development. Anal Bioanal Chem 402:3071–3089
Ghobril C et al (2012) Dendrimers in nuclear medical imaging. New J Chem 36:310–323
Kolhe P et al (2003) Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int J Pharmaceut 259:143–160
Sekowski S et al (2011) Interaction of polyamidoamine (PAMAM) succinamic acid dendrimers generation 4 with human serum albumin. Spectrochim Acta A 81:706–710
He J et al (2003) Pharmacokinetics in mice of four oligomer-conjugated polymers for amplification targeting. Cancer Biother Radio 18:941–947
Thor A et al (1986) Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Res 46:3118–3124
Loy TS et al (1993) Reactivity of B72.3 with adenocarcinomas. An immunohistochemical study of 476 cases. Cancer-Am Cancer Soc 72:2495–2498
Cai L et al (2021) Research on preparation and in vitro evaluation of the dendrimer-peptide nuclear acid conjugate for amplification pretargeting. J Label Compd Rad 64:428–439
Harris SG et al (2003) Growth of endothelial cells on microfabricated silicon nitride membranes for an in vitro model of the blood-brain barrier. Biotechnol Bioproc E 8:246–251
Zhang MR et al (2007) [F-18]Fluoroalkyl agents: Synthesis, reactivity and application for development of PET ligands in molecular imaging. Curr Top Med Chem 7:1817–1828
Mukai H et al (2014) Quantitative evaluation of the improvement in the pharmacokinetics of a nucleic acid drug delivery system by dynamic PET imaging with F-18-incorporated oligodeoxynucleotides. J Control Release 180:92–99
Stein CA et al (2005) Antisense strategies for oncogene inactivation. Semin Oncol 32:563–572
Lendvai G et al (2008) Biodistribution of Ga-68-labeled LNA-DNA mixmer antisense oligonucleotides for rat Chromogranin-A. Oligonucleotides 18:33–49
Lendvai G et al (2005) Biodistribution of Ga-68-labelled phosphodiester, phosphorothioate, and 2 ‘-O-methyl phosphodiester oligonucleotides in normal rats. Eur J Pharm Sci 26:26–38
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 11705268).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, L., Wang, H., He, S. et al. Synthesis and in vivo evaluation of 18F-cPNA and Dendrimer-PNA conjugate for amplification pretargeting. J Radioanal Nucl Chem 331, 2895–2902 (2022). https://doi.org/10.1007/s10967-022-08289-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08289-y