Abstract
This study presents a rapid and novel sequential separation strategy based on extraction chromatography for determining 90Sr, 210Pb and 210Po in drinking water samples. It involves the use of Sr resin for the separation and then liquid scintillation counting and alpha spectrometry for the determination. The experimental results obtained showed that the proper acidic solution to quantitatively retain the aforementioned radionuclides is 3 M HNO3. The optimum eluents were determined for obtaining quantitative recoveries (70–80%) of 90Sr, 210Pb and 210Po. The method was validated with intercomparison water samples and is satisfactory in terms of minimum detectable activities, which are 50% lower than that established in RD 314/2016.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water intended for human consumption is generally treated for potabilization, with various parameters being routinely controlled to ensure its quality and safe distribution to the population as described in European Directive (EU) 2020/2184 [1]. Of these parameters, one of the most important is the evaluation of the drinking water’s radiological quality.
In Spain, control of the radioactive substances present in water intended for human consumption and their screening level requirements is performed in accordance with Royal Decree 314/2016 [2], which was transposed from Council Directive 2013/51/Euratom [3]. The Decree requires the total indicative dose (ID) to be assessed, and to this end the activity of certain artificial and natural radionuclides needs to be determined. Different radionuclide activities have to be determined in different situations, such as when the gross alpha index activity exceeds 0.1 Bq L−1. The present study focuses on the activity determination of three of the radionuclides specified in the legislation: 90Sr, 210Pb and 210Po.
In the literature there are various approaches in which these radionuclides have been determined in environmental water samples [4,5,6]. These strategies generally include a radiochemical separation, which is mandatory as sample pretreatment to avoid measurement interferences [5, 7, 8]. The authors of these approaches have usually focused on determining a single radionuclide or in some cases two. However, it is important to stress that the development of sequential radiochemical strategies to separate more radionuclides is of great interest, since it could be very useful in shortening the analysis time. The development of extraction chromatographic resins has contributed notably to enabling radionuclide separation/isolation.
Sr resin has become one of the most popular resins for isolating 90Sr, 210Pb and 210Po prior to their measurement. In the literature, as can be seen in Table 1, there are a number of Sr resin based strategies that individually separate one of these three radionuclides [8,9,10] or even isolate two of them – e.g. 90Sr and 210Pb [11,12,13], 90Sr and 210Po [14] or 210Pb and 210Po [5, 7, 15,16,17,18,19,20,21] – in different kinds of matrices. The table shows the load sample, resin conditioning media and radionuclide elution media used, these being the main parameters that may have an influence on recovery.
It can be observed that acidic conditions are commonly used in the conditioning and sample loading steps. Authors generally use HNO3 or HCl at different concentrations for this purpose. In the case of 90Sr, its affinity with the resin reaches maximum values at between 3 and 8 M HNO3 [9]. However, as previously stated in the literature [22], hydrochloric acid media are useless in terms of strontium extraction because de-solvating strontium chloride neutral species is more difficult than de-solvating neutral nitrate species formed using nitric acid.
As regards 210Pb, this radionuclide presents a high retention in nitric acid (0.1 – 8 M) and hydrochloric acid (0.1 – 2 M) media [18, 23]. Similarly, 210Po is highly retained in 1 M HNO3 [23], despite the fact that high recoveries were also achieved in other conditions, such as stronger nitric acid concentrations (8 M HNO3) and in a wide range of HCl concentrations [5]. It can therefore be concluded that both the composition of the conditioning solvent and the sample loading solution are important because they have a notable effect on the selectivity and retention capacity of each radionuclide on the resin.
The aim of this study is to present for the first time a strategy to sequentially separate and then determine three radionuclides–90Sr, 210Pb and 210Po–using the Sr resin. To this end the various experimental conditions that may have an effect on the efficacy of the retention of the radionuclides in that resin are evaluated. This approach will be useful in minimizing the turnaround time needed to separate and determine the radionuclides and can therefore be used in situations that call for a quick response, especially when determining those radionuclides that contribute to the indicative dose estimation (RD 314/2016) in drinking water samples. Finally, the method is tested and validated by analysing water samples from intercomparison exercises.
Experimental part
Reagents and instruments
All the chemical reagents and solutions used in this study were of analytical grade. HCl (35%) and HNO3 (65%) were supplied by J.T. Baker (Deventer, Holland). Ammonium oxalate and ascorbic acid were supplied by Chem-Lab (Zedelgem, Belgium) and Panreac (Darmstatd, Germany). Strontium nitrate and lead nitrate were supplied by Fluka Chemie GmbH (Switzerland) and Probus (Barcelona, Spain) respectively.
Sr resin (100–150 µm, 2 mL columns) and a 12-hole vacuum box were supplied by TrisKem International (France) and were used for the radiochemical separation of 90Sr, 210Pb and 210Po. The extractant used in Sr resin is a crown-ether (4,4’(5’)-di-tert-butylcyclohexane-18-crown-6) diluted in 1-octanol.
The liquid scintillation (LS) cocktail, Optiphase HiSafe 3, and polyethylene (PE) scintillation vials used to prepare the 90Sr and 210Pb samples were supplied by PerkinElmer (Waltham, USA) and Sarstedt (Nümbrecht, Germany) respectively. To determine these radionuclides an ultra-low-background Quantulus 1220™ liquid scintillation spectrometer (PerkinElmer) was used. It was equipped with three sample tray tables with 20 positions and the EASY view spectrum analysis programme.
210Po was auto-deposited on silver disks supplied by Goodfellow (Huntingdon, United Kingdom) and determined by alpha spectrometry (EG&G ORTEC, 676 Model, USA), which includes an ion-implanted silicon detector (ORTEC, size: 450 mm2; alpha resolution: 25 keV FWHM at 5.48 MeV of 241Am) in a vacuum chamber (Edwards, Model E2M8), a detector bias supplier, a preamplifier, a linear preamplifier and a multichannel pulse height analyser.
The standard solutions of 90Sr/90Y, 210Pb/210Bi/210Po and 209Po used for the method optimization were provided by CIEMAT (Madrid, Spain) and Eckert & Ziegler (Valencia, California). The certified nominal activity for 90Sr/90Y was 246.7 ± 0.3 Bq g−1, for 210Pb/210Bi/210Po it was 580 ± 10 Bq g−1 and for 209Po it was 100 ± 15 Bq g−1.
An inductively coupled plasma mass spectrometry (ICP-MS) Elan DRC-e (Perkin Elmer, Spain) was used to calculate the recovery of the sequential separation process for the stable strontium and lead. The system was fitted with a Scott spray chamber and a crossflow nebulizer (Perkin Elmer, Spain). 71 Ga and 205Tl were used as internal standards to compensate for instrument drift between runs.
Batch studies
The chromatographic capacity factor (k´) for the Sr resin for the radionuclides under study was determined. The procedure used was as follows.
First, 10 mL solutions containing 0.5 mL of Sr(II) and Pb(II) solutions (1000 ppm respectively) and 1 mL of 209Po (5039 Bq L−1) at the appropriate HNO3 molarity (3 M) were shaken for 24 h with 0.15 g of Sr resin. Blank samples were also measured. The samples were then filtered using a syringe and a 0.45 µm cellulose filter. The Sr(II) and Pb(II) present in the nitric phase were determined by ICP-MS, while 209Po activity was determined by LSC.
From this batch study, the weight distribution value (Dw) of the Sr resin can be calculated by determining the radionuclide activity in the case of 209Po or the element concentration in the case of Sr(II) and Pb(II), in solution before and after their contact with the Sr resin. This is done using Eq. 1 [24]:
where A0 is the initial element activity (Bq mL−1) or concentration (mg L−1), As is the final element activity (Bq mL−1) or concentration (mg L−1) after being in contact with the Sr resin, V is the sample volume (mL), and m is the mass of Sr resin used in the batch experiment (g).
Dw can then be used to obtain the chromatographic capacity factor (k’ in g mL−1), which can be calculated using Eq. 2 [24]:
where f is the specific conversion factor for Dw to k’ for the Sr resin and has a value of 2.17 [25].
Procedure
The procedure used to sequentially separate and determine 90Sr, 210Pb and 210Po in water samples is composed of three steps: pretreatment of the water sample, radionuclide separation using the Sr resin, and finally measurement of 90Sr and 210Pb with a liquid scintillation counter and of 210Po with an alpha spectrometer.
First of all a volume of 500 mL of water sample was evaporated to dryness with a sand bath, under controlled temperature to avoid polonium volatilization [5], after adding the corresponding tracers (0.5 mg of Sr(II), 0.5 mg of Pb(II) and 0.5 mL of 209Po tracer at 23.46 ± 0.49 Bq L−1). The dry residue was then re-dissolved to a final volume of 10 mL with 3 M HNO3 and loaded through a 2 mL column of Sr resin.
The sequential separation of 90Sr, 210Pb and 210Po was performed following the steps of the complete schema shown in Fig. 1 and Table 2. First, the 2 mL columns of Sr resin were connected to a 12-hole vacuum box with a peristaltic pump and to a funnel. The conditioning step was then performed with 10 mL of a solution of 3 M HNO3 before loading the sample solution with a flow rate of 1 mL min−1, in accordance with the protocol (steps I and II in Table 2). The retained polonium was then eluted with 10 mL of 8 M HNO3 (step III). Afterwards the strontium was eluted with 10 mL of 0.05 M HNO3 (step IV), and the lead was eluted with 10 mL of 0.06 M (NH4)2C2O4 (step V).
Once the sequential separation had been carried out, the activity of 90Sr and 210Pb were determined using a low background Quantulus 1220™ liquid scintillation spectrometer. In the case of 90Sr, samples were measured by selecting the high-energy beta measurement mode. The ratio between the sample volume and the LSC cocktail volume was 8:12. The sample was stored for 15 days in order to allow for the ingrowth of 90Y and the establishment of the 90Y/90Sr radioactive equilibrium. For 210Pb, the alpha/beta measurement mode with pulse shape analysis (PSA) was used, set at a value of 95 [26]. In this case the volume ratio between the sample and the scintillation cocktail was 4:16. 210Pb was measured immediately after separation to avoid the growth of daughter radionuclides. The counting time for both radionuclides was 1000 min (2 cycles of 500 min). Sample aliquots were also taken to determine stable strontium and lead content by means of ICP-MS and to calculate the recovery of the sequential separation process. The 90Sr and the 210Pb can be calculated using Eq. 3:
where A is the radionuclide activity (Bq L−1), Rg is the gross count rate (s−1), R0 is the background count rate in (s−1), ε is the counting efficiency, η is the chemical yield, V is the sample volume (L), fa is the aliquot correction factor (the volume of the aliquot of Pb or Sr fraction divided by the volume of total eluted fraction) and Kw is the waiting time correction factor.
210Po was auto-deposited on a silver disk following a modified procedure previously published by our laboratory [27]. Auto-deposition was performed for 3 h at 85 °C in an 80 mL 2 M HCl solution under continuous stirring. Samples were counted for 300,000 s with a chamber pressure of 10–2 Torr, and the radiochemical recovery was obtained by determining the 209Po tracer activity. The detection efficiencies, which were derived from the detector calibration using a stainless steel planchet with certified activities of 233U, 239/240Pu and 241Am, ranged between 17.77% and 17.92%.
Intercomparison samples
Two drinking water samples from intercomparison exercises organized by the Consejo de Seguridad Nuclear (CSN) (CSN-CIEMAT 2019) and the International Atomic Energy Agency (IAEA) (IAEA-TEL-2020–03) were analysed in order to test the proposed method in two activity ranges. The CSN-CIEMAT 2019 sample was a deionized water simulating drinking water with low-level activity of 90Sr, 210Pb and 210Po, among other radionuclides, with activity concentrations of 7.54 ± 0.50 Bq L−1, 0.39 ± 0.30 Bq L−1 and 0.253 ± 0.060 Bq L−1 respectively. The IAEA-TEL-2020–03 was a tap water from Seibersdorf, Austria, with high-level activity values of 210Pb and 210Po, among other radionuclides, with activity concentrations of 905 ± 17 Bq L−1 and 921 ± 20 Bq L−1 respectively.
Results and discussion
In the following section we present a novel sequential strategy for the separation of 90Sr, 210Pb and 210Po. This approach will be very useful to provide a rapid response in situations of emergency, for example when these particular radionuclides need to be determined in drinking water, since they are some of the contributors to the ID estimation. The evaluation of the radionuclide retention in a Sr resin is performed using the chromatographic capacity factor to evaluate different experimental conditions. Lastly, under the optimum conditions found for the selective isolation of the three radionuclides evaluated, the method was validated with intercomparison samples.
Batch study of 90Sr, 210Pb and 210Po in Sr resin
In order to perform the batch study, different concentrations of HNO3 in the range from 1 to 4 M were employed to obtain optimal 90Sr, 210Pb and 210Po retention in the Sr resin. The results can be seen in Fig. 2, which shows the k’ values for all three elements when they are together in the same sample, simulating the worst scenario in which possible influence effects by competition between elements may occur.
It can be seen that strontium presented an increase in retention when a higher nitric acid concentration was used. For this radionuclide, k’ values of approximately 20 g mL−1 were obtained for an HNO3 concentration of 1 M and 60 g mL−1 at an acid concentration of 4 M . These values are in line with those published previously by TrisKem International [23], where the k’ value drops from almost 90 g mL−1 at 8 M HNO3 to less than 1 g mL−1 at 0.05 M HNO3. Taking this into account and looking at Fig. 2, it can be concluded that the presence of 0.5 mg of Pb(II) and 0.01 Bq of 209Po has no effect on strontium resin retention.
As regards Pb, and as shown in Fig. 2, this element presented a good retention in all the acid concentrations tested. Indeed, its k’ value slightly decreased from average values of 760 to 670 g mL−1 with the increase in the acid concentration. This is in line with results previously obtained by other authors such as Kong et al. [18], who developed a method to determine 210Bi, 210Pb and 210Po in seafood samples by using the same resin. In the case of 210Pb, these same authors reported that it was retained in HNO3 media, but the higher the nitric concentration, the lower the 210Pb Sr resin affinity.
210Po presented a slight better retention in 3 M HNO3 with a k’ value of 11 g mL−1 compared to the other acid molarities tested. Note that in 4 M HNO3, 210Po retention decreased. Although TrisKem reported better results in terms of k’ for Po(IV) in a lower acidic range (0.5–1 M HNO3) than those obtained in our study [5], a number of published studies in the literature find results in line with ours [10, 14, 28]. In those cases the best performance for Po retention was also achieved for higher nitric concentrations to retain and selectively separate 210Po from the matrix and from other radionuclides. For example, Hurtado-Bermúdez et al. used a high nitric acid concentration, 8 M, in a study focusing on the separation of 210Po and 90Sr and their determination in the soft tissue of seafood samples such as oysters, mussels and sea urchins from the southern Spanish Atlantic coast [14].
Finally, taking into account all the results for k’ for the three radionuclides under study, 3 M was the chosen molarity of the nitric acid solution for the sample loading and conditioning in the sequential separation procedure, being a compromise between all the values obtained and thus ensuring the optimal retention of each radionuclide in the Sr resin.
Optimization of Sr resin separation parameters
An optimization of the different variables that affect the proposed strategy was carried out, including the volumes and concentrations of different reagents. Both the elution step and the conditions used were optimized, leading to good recoveries in a previous study performed in our laboratory [11]. 8 M HNO3, 0.05 M HNO3 and 0.06 M ammonium oxalate were used to elute 210Po, 90Sr and 210Pb respectively. The volume of 90Sr and 210Pb elution solution (10 mL) was the same we used in our laboratory to perform intercomparison exercises, obtaining recoveries of 70–80%. This volume is also in line with Dalencourt et al. [29], who reported efficient Pb elution with 10 mL of ammonium oxalate. In the case of 210Po, a total volume of 10 mL of 8 M HNO3 was collected in 2 mL fractions to determine the minimum stripping volume required. The results obtained showed that, for 10 mL, over 80% of the initial polonium can be eluted.
In addition to optimizing the Sr resin separation parameters, all the elution fractions and wastes were analysed to determine the presence of 90Sr, 210Pb and 210Po and to check there were not interferences in the measurements. As an example, 210Po not eluted (approximately 20%) remained in the waste of the sample loading, so no interference in the 90Sr measurement is expected.
Method validation
This novel sequential separation of 90Sr, 210Pb and 210Po was validated via an analysis in triplicate of water samples from two different intercomparison exercises organized by CSN-CIEMAT in 2019 and the IAEA in 2020. These samples contained 90Sr, 210Pb and 210Po in different ranges of activity concentrations.
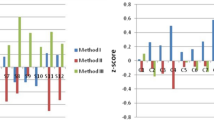
Table 3 shows the mean activity values obtained for 90Sr, 210Pb and 210Po using the method developed in the present study and the certified values reported by the intercomparison exercise organizers. The values obtained generally agreed with the established values except for the IAEA-TEL-2020–03 sample in which 210Po was underestimated, probably due to a problem in connection with the stability of the sample. Nevertheless, the z-score value–which is a standard measure of laboratory systematic error calculated using the certified value and standard deviation of the exercise–was − 1.4, indicating that the result obtained was satisfactory. In the case of the CSN-CIEMAT 2019 sample, the organizer stated that the 210Pb activity in the sample was not known. The certified value shown in the table is the average value obtained from all those reported by participants. The participants’ results were widely dispersed and the CSN-CIEMAT communicated that the 210Pb activity concentration was not evaluable. Bearing all this in mind, the 210Pb activity concentration obtained agreed with the mean value reported by the CSN-CIEMAT.
The method’s reproducibility, expressed as relative standard deviation (RSD), was evaluated by determining 90Sr, 210Pb and 210Po activity concentrations on three consecutive days. The values obtained are also shown in Table 3.
Minimum detectable activity (MDA) is an important parameter for estimating the suitability of a method, and this was determined using the Curie definition. Table 4 shows the MDA calculated in the present study for a volume of 500 mL of water samples. It can be seen that the sequential method is suitable for determining 90Sr, 210Pb and 210Po in fast response situations that require an ID such as MDA to be calculated for 90Sr, 210Pb and 210Po. These were 97.5%, 50% and 60% lower respectively than those established in RD 314/2016 [2].
The current method also showed improvements in the MDAs of 90Sr and 210Pb compared to the conventional methods used in the laboratory as regards the quantity of sample volume needed and the time spent on sample pretreatment and radionuclide separation. The sample volume required in the present method is three times lower than that required to determine the three radionuclides by conventional methods. For example, in our laboratory a volume of 4 L is required to determine 210Po in water samples [27]. We could also save around 24 h in terms of sample pretreatment.
As can be observed in Table 5, different figures of merit such as sample volume, analysis time and MDA can be compared between the present study and other methods previously published in the literature that have focused on determining 90Sr, 210Pb and 210Po in water samples. In the case of MDA, the results obtained in this study are comparable with others that require higher water volumes to perform the radionuclide determination. While the methodology presented by Villa et al. [17] was able to achieve the lowest MDA, this was done by pretreating large water volumes (30 L), which is more time-consuming and tedious. Lluch et al. [30] developed a method based on PS resin that combined the separation and detection steps. Although this strategy had only been applied on spiked environmental water samples, the MDA presented did not represent a water sample with environmental activity levels. Finally, most of the studies presented in the literature do not specify how long it takes for the analysis to be carried out. However, one might expect that, due to the large volume samples handled, it would require high turnaround times. The method we present in this study took about 4 h to separate the radionuclides, which is suitable for rapid response situations.
Conclusions
The proposed strategy for sequentially separating and determining 90Sr, 210Pb and 210Po using an extraction chromatographic Sr resin was successfully applied for the first time. The sequential separation of these three radionuclides was performed using 3 M HNO3 as the optimal solution to condition and load the sample and determine good retention of the radionuclides under study.
It was applied to two intercomparison water samples and obtained good reproducibility values and low minimum detection activities. The present approach could therefore be an interesting alternative for separating and determining 90Sr, 210Pb and 210Po in water samples compared to conventional methods. It has the advantage in terms of rapid response and would be useful in emergency situations.
References
Diario Oficial de la Unión Europea (2020) Directiva (UE) 2020/2184 del Parlamento Europeo y del Consejo de 16 de diciembre de 2020 relativa a la calidad de las aguas destinadas al consumo humano
RD 314/2016 (2016) Real Decreto 314/2016, de 29 de julio, por el que se modifican el Real Decreto 140/2003, de 7 de febrero, por el que se establecen los criterios sanitarios de la calidad del agua de consumo humano, el Real Decreto 1798/2010, de 30 de diciembre, por el que se regula la explotación y comercialización de aguas minerales naturales y aguas de manantial envasadas para consumo humano, y el Real Decreto 1799/2010, de 30 de diciembre, por el que se regula el proceso de elaboración y comercialización de aguas preparadas envasadas para el consumo humano. Boletín Oficial del Estado, número 183. Madrid, 30 de julio de 2016
European Commission (2013) Council Directive 2013/51/EURATOM of 22 October 2013 laying down requirements for the protection of the health of the general public with regard to radioactive substances in water intended for human consumption
Szabo Z, Stackelberg PE, Cravotta CA (2020) Occurrence and geochemistry of Lead-210 and Polonium-210 radionuclides in public-drinking-water supplies from principal aquifers of the united states. Environ Sci Technol 54:7236–7249. https://doi.org/10.1021/acs.est.0c00192
Thakur P, Ward AL (2020) 210Po in the environment: insight into the naturally occurring polonium isotope. J Radioanal Nucl Chem 323:27–49. https://doi.org/10.1007/s10967-019-06939-2
Shao Y, Yang G, Tazoe H et al (2018) A review of measurement methodologies and their applications to environmental 90Sr. J Environ Radioact 192:321–333. https://doi.org/10.1016/j.jenvrad.2018.07.013
El Afifi EM, Borai EH (2006) Performance characteristics of sequential separation and quantification of lead-210 and polonium-210 by ion exchange chromatography and nuclear spectrometric measurements. J Environ Qual 35:568–574. https://doi.org/10.2134/jeq2005.0223
Vajda N, Kim CK (2010) Determination of radiostrontium isotopes: a review of analytical methodology. Appl Radiat Isot 68:2306–2326. https://doi.org/10.1016/j.apradiso.2010.05.013
Dianu AM, Dobrin RI (2020) Separation and quantification of 90Sr from ion-exchange resin radioactive waste: methods and techniques of analysis. Radiochim Acta 108:627–640. https://doi.org/10.1515/ract-2019-3213
Hurtado-Bermúdez S, Mas JL (2019) Determination of 210Po in low-level wild bilberries reference material for quality control assurance in environmental analysis using extraction chromatography and α-particle spectroscopy. Radiochim Acta 108:99–103. https://doi.org/10.1515/ract-2019-3141
Mola M, Avivar J, Nieto A et al (2014) Determination of 90Sr and 210Pb in sludge samples using a LOV-MSFIA system and liquid scintillation counting. Appl Radiat Isot 86:28–35. https://doi.org/10.1016/j.apradiso.2013.11.123
Nonova T, Tosheva Z (2016) 90Sr, 210Pb, 210Po and Ra isotopes in marine macroalgae and mussel Mytilus galloprovincialis from the Bulgarian Black Sea zone. J Radioanal Nucl Chem 307:1183–1194. https://doi.org/10.1007/s10967-015-4502-x
Landstetter C, Sinojmeri M, Katzlberger C, Achatz A (2014) Determination of 90Sr and 210Pb in freshwater fish in Austria. J Radioanal Nucl Chem 299:787–790. https://doi.org/10.1007/s10967-013-2700-y
Hurtado-Bermúdez S, Mas JL, Villa-Alfageme M (2017) A sequential determination of 90Sr and 210Po in food samples. Food Chem 229:159–164. https://doi.org/10.1016/j.foodchem.2017.02.077
Ozden B, Vaasma T, Kiisk M, Tkaczyk AH (2017) A modified method for the sequential determination of 210Po and 210Pb in Ca-rich material using liquid scintillation counting. J Radioanal Nucl Chem 311:365–373. https://doi.org/10.1007/s10967-016-4984-1
Vajda N, LaRosa J, Zeisler R et al (1997) A novel technique for the simultaneous determination of 210Pb and 210Po using a crown ether. J Environ Radioact 37:355–372. https://doi.org/10.1016/S0265-931X(95)00059-J
Villa-Alfageme M, Mas JL, Hurtado-Bermudez S, Masqué P (2016) Rapid determination of 210Pb and 210Po in water and application to marine samples. Talanta 160:28–35. https://doi.org/10.1016/j.talanta.2016.06.051
Kong X, Yin L, Ji Y (2021) Simultaneous determination of 210Pb and 210Po in seafood samples using liquid scintillation counting. J Environ Radioact 231:106553. https://doi.org/10.1016/j.jenvrad.2021.106553
Miura T, Hayano K, Nakayama K (1999) Determination of 210Pb and 210Po in environmental samples by alpha ray spectrometry using an extraction chromatographic resin. Anal Sci 15:23–28. https://doi.org/10.2116/analsci.15.23
Fan F, Liu H, Liang J et al (2020) Rapid separation of Po-210 from Pb-210 based on the usage of a commercial Sr-Specific chromatographic resin. J Environ Radioact 211:106083. https://doi.org/10.1016/j.jenvrad.2019.106083
Pan LJ, Yu GB, Chen Z et al (2018) A modified sampling preparation method for rapid determination of Pb-210 radioactivity in plants in China using crown ether and liquid scintillation counting of beta particles. J Radioanal Nucl Chem 317:565–570. https://doi.org/10.1007/s10967-018-5919-9
McLain DR, Liu C, Sudowe R (2018) Using Sr Resin with mixed acid matrices. J Radioanal Nucl Chem 316:485–490. https://doi.org/10.1007/s10967-018-5778-4
Eichrom Technologies (2021) Sr resin. https://www.eichrom.com/eichrom/products/sr-resin/. Accessed 2 Mar 2021
Horwitz EP (2018) The Capacity Factor (k’): How it is measured and what it does not tell you. https://www.eichrom.com/wp-content/uploads/2018/02/the_capacity_factor.pdf. Accessed 2 Mar 2021
Triskem International (2015) Sr resin. Product Sheet. https://www.triskem-international.com/scripts/files/5f463447ad4026.94629022/PS_SR-Resin_EN_160927.pdf. Accessed 2 Mar 2021
Palomo M, Villa M, Casacuberta N et al (2011) Evaluation of different parameters affecting the liquid scintillation spectrometry measurement of gross alpha and beta index in water samples. Appl Radiat Isot 69:1274–1281. https://doi.org/10.1016/j.apradiso.2011.04.020
Fonollosa E, Peñalver A, Aguilar C, Borrull F (2015) Polonium-210 levels in different environmental samples. Environ Sci Pollut Res 22:20032–20040. https://doi.org/10.1007/s11356-015-5158-3
Horwitz EP, Chiarizia R, Dietz L (1992) A novel strontium-selective extraction chromatographic resin. Solvent Extranction Ion Exch 10:313–336. https://doi.org/10.1080/07366299208918107
Dalencourt C, Chabane MN, Tremblay-Cantin JC, Larivière D (2020) A rapid sequential chromatographic separation of U- and Th-decay series radionuclides in water samples. Talanta 207:120282. https://doi.org/10.1016/j.talanta.2019.120282
Lluch E, Barrera J, Tarancón A et al (2016) Analysis of 210Pb in water samples with plastic scintillation resins. Anal Chim Acta 940:38–45. https://doi.org/10.1016/j.aca.2016.08.004
Acknowledgements
The authors would like to thank the Consorci d’Aigües de Tarragona (CAT) for their invaluable cooperation.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez, J., de los Cobos, M., Peñalver, A. et al. A fast strategy to sequentially separate and determine 90Sr, 210Pb and 210Po in water samples using Sr resin. J Radioanal Nucl Chem 331, 629–637 (2022). https://doi.org/10.1007/s10967-021-08093-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-08093-0