Abstract
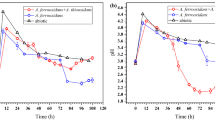
Low-grade uranium ore, which was challenging to be treated economically by conventional methods, can be treated by uranium leaching by bacteria. However, high uranium concentration will lead to the death of bacteria. This work researched the oxidative leaching performance of bacteria (Acidithiobacillus ferrivorans with uranium resistance) under different iron valence conditions. Oxidative leaching of UO2 by bacteria from energy substrates with varying states of the valence of iron ions was studied through an Acidithiobacillus ferrivorans oxidation model. The results showed that the probability of pyrite being oxidized by the Acidithiobacillus ferrivorans system was 98.05% under the condition of Fe2+, while it was 87.30% exposed to Fe3+. In addition, the leaching rate of UO2 by the Acidithiobacillus ferrivorans & Fe2+ system was 94.70%. Under the same conditions, while adding 1 g/L pyrite, the leaching rate of UO2 increased to 99.96%. This study revealed the oxidative leaching mechanism of UO2 and provided a new theoretical basis for the bacterial leaching of uranium.









Similar content being viewed by others
References
Wei J (2020) Research progress of bio-metallurgy and its application. J East China Univ Technol 03:48–53
Li G, Zhou Y, Zhao K, Liu J, Lingling Xu, Liao Z, Ni F (2021) Research progress on leaching mineral technology of sandstone-type uranium deposits. Non-ferr Metals 08:9–19
Silverman MP, Ehrlich HL (1964) Microbial formation and degradation of minerals. J Adv Appl Microbiol 6(6):153–206
Crundwell FK (2003) How do bacteria interact with minerals. J Hydrometallurgy 71(1–2):75–81
Ilyas S, Kim MS, Lee JC (2018) Integration of microbial and chemical processing for sustainable metallurgy. J Chem Technol Biotechnol 93:305–350
Wang Y, Li G, Ding D, Zhou Z, Deng Q, Hu N, Tan Y (2013) Uranium leaching using mixed organic acids produced by Aspergillus niger. J Radioanal Nucl Chem 298(2):769–773
Shuang T, Xiaoyu W (2015) Cold-adapted microorganisms and applications in sewage treatment. J Bull Sci Technol 07:229–236
Li Y, Yun Y, Jiao R (2007) Study on the psychrotrophic bacteria to treat domestic cryogenic sewage. J Appl Sci Technol 34(004):63–66
Hallberg KB, Gonzalez-Toril E, Johnson DB (2010) Acidithiobacillus ferrivorans, sp. Nov. facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 14(1):9–19
Barahona S, Dorador C, Zhang R, Aguilar P, Sand W, Vera M, Remonsellez F (2014) Isolation and characterization of a novel Acidithiobacillus ferrivorans strain from the Chilean Altiplano: attachment and biofilm formation on pyrite at low temperature. J Res Microbiol 165(9):782–793
Peng C, Qingliang W, Eming H (2018) Growth characteristics of Acidithiobacillus ferrivorans and its immobilization culture. Metal Mine 3:90–96
Zeng W, Peng Y, Peng T, Nan M, Chen M, Qiu G, Shen L (2020) Electrochemical studies on dissolution and passivation behaviour of low-temperature bioleaching of chalcopyrite by Acidithiobacillus ferrivorans YL15. Miner Eng 155:106416
Peng T, Chen L, Wang J, Miao J, Shen L, Yu R, Gu G, Qiu G, Zeng W (2019) Dissolution and passivation of chalcopyrite during bioleaching by Acidithiobacillus ferrivorans at low temperature. Minerals 9(6):332
Tran Tam TTT et al (2017) Comparative genome analysis provides insights into both the lifestyle of Acidithiobacillus ferrivorans strain CF27 and the chimeric nature of the iron-oxidizing Acidithiobacilli genomes. J Front Microbiol 8:1009
Ge Y, Zhou Z, Fang X (2021) Effects of different types of pyrite on uranium bioleaching behavior. (01):48-55. http://yayl.bgrimm.cn
Juan L (2015) Supergene oxidation of pyrite and the study for microbial oxidation mechanism. School of Earth Sciences and Engineering, Nanjing University
Wang M, Liang J, Zeng W (2018) Bioleaching of tin-rich polymetallic sulphide ore by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. J. Nonferr. Metals Sci. Eng. 9(03):76–83
Emergasso CS, Galleguillos P, Escudero L et al (2005) Molecular characterization of microbial populations in a low-grade copper ore bioleaching test heap. J Hydrometallurgy 80(4):241–253
Xue H (2017) Study on the bioleaching and mechanism of metals from nickel-molybdenum ore tailings with Acidthiobacillus ferrooxidans. Guizhou University
Chen H, Duorui Z, Zhenyuan N, Lei Z, Lili Z, Hongying Y, Jinlan X (2020) Study on the transformation of iron/arsenic/sulfur species in the process of arsenic pyrite bioleaching based on spectral analysis. J Spectrosc Spectr Anal 40(03):934–940
Tang Y (2020) Dissolved organic matter mediates the phase transition of jarosite and the redistribution mechanism of loaded arsenic. South China University of Technology
Fan C (2019) Redistribution mechanism of previously-bound heavy metals during the Fe(II)-induced recrystallization of schwertmannite. South China University of Technology
Mikutta R, Lorenz D, Guggenberger G, Haumaier L, Freund A (2014) Properties and reactivity of Fe-organic matter associations formed by coprecipitation versus adsorption: clues from arsenate batch adsorption. J Geochim Cosmochim Acta 144:258–276
Acknowledgements
Thank you very much for the financial support of the Ministry of Science and Technology of China. This material is based on the work supported by the [National Ministry of Science and Technology Key R&D Program Project], the Project Number: 2019YFC1907701.
Author information
Authors and Affiliations
Contributions
YD: Analyzed data, XPS data, XRD data, SEM data, a manuscript written and edited. Wei Hou: XRD data and analysis. XW: Designed Model, Measured the element of Fe. YZ: FTIR data and analysis. QW: Designed the experiment. HW: Manuscript edited.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Y., Hou, W., Wei, X. et al. Mechanism analysis on synergistic leaching of uranium from pyrite oxidized by Acidithiobacillus ferrivorans. J Radioanal Nucl Chem 330, 951–961 (2021). https://doi.org/10.1007/s10967-021-08026-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-08026-x