Abstract
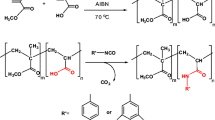
The aim of this study is the synthesis of a novel 99mTc-labeld graft polymer and the biological evaluation of its in vitro and in vivo properties. To this end, a L-proline-graft-poly(HEMA) was prepared and labeled with 99mTc. The radiochemical yield of approximately the 99mTc-labeled compound amounted to 97 ± 2.3%. The cytotoxicity test revealed no cytotoxic effect after a 24- and 48-h incubation. The results of the hemolysis test showed that hemolysis was non-toxic with an effect level of less than 2%. Subsequently, the biodistribution in healthy rats was determined. High accumulation of the polymer was observed in the pancreas, thyroid and prostate.













Similar content being viewed by others
References
Yang B, Zhang F, Yuan WL, Du L, Jiang XJ, Javadzadeh Y, Araneda R (2021) Preparation of isorhamnetin nanoparticles and their targeting efficiency to nasopharynx cancer. J Nanosci Nanotechnol 21:1293–1299
Zheng YY, Hong XQ, Wang JT, Feng LB, Fan TJ, Guo R, Zhang H (2021) 2D Nanomaterials for Tissue Engineering and Regenerative Nanomedicines: Recent Advances and Future Challenges. Adv Healthc Mater 10(7):2001743
Rabiee N, Ahmadi S, Fatahi Y, Rabiee M, Bagherzadeh M, Dinarvand R, Bagheri B, Zarrintaj P, Saeb MR, Webster TJ (2020) Nanotechnology-assisted microfluidic systems: from bench to bedside. Nanomedicine 16:237–258
Hayran O (2019) New medical technologies and ethical issues. J Biotechnol 3:54–60
Sahoo SK, Parveen S, Panda JJ (2007) The present and future of nanotechnology in human health care. Nanomed-Nanotechnol 3:20–31
Inanan T, Tüzmen N, Akgöl S, Denizli A (2016) Selective cholesterol adsorption by molecular imprinted polymeric nanospheres and application to GIMS. Int J Biol Macromol 92:451–460
Vermeulen K, Vandamme M, Bormans G, Cleeren F (2019) Design and challenges of radiopharmaceuticals. Seminars in nuclear medicine 49(5):339–356
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Sukumaran J, Hanefeld U (2005) Enantioselective C-C bond synthesis catalysed by enzymes. Chem Soc Rev 34:530–542
Faber K (2000) Biotransformations in Organic Chemistry. Springer, New York
Saboury B, Morris M, Cao Q (2018) Determination of cis-4-[18F][1]fluoro-L-proline (18F-FP) normal biodistribution in experiments optimized for hepatic uptake using dynamic and static PET/CT imaging. J Nucl Med 59:1235
Ponrasu T, Jamuna S, Mathew A, Madhukumar KN, Ganeshkumar M, Iyappan K, Suguna L (2013) Efficacy of L-proline administration on the early responses during cutaneous wound healing in rats. Amino Acids 45:179–189
Bhat A, Smith B, Dinu CZ, Guiseppi-Elie A (2019) Dataset on hydrophobicity indices and differential scanning calorimetry thermograms for poly (HEMA)-based hydrogels. Data in brief. 24:103891
Andrade, J. D. (Ed.) (1976) Hydrogels for medical and related applications. American Chemical Society
Montheard JP, Chatzopoulos M, Chappard D (1992) 2-hydroxyethyl methacrylate (HEMA): chemical properties and applications in biomedical fields. Polym Rev (Phila Pa) 32:1–34
Ratner BD, Hoffman AS, Schoen FJ (2004) Orthopedic materials. Application of materials in medicine, biology and artificial organs. Biomaterials Science: An Introduction to Materials in Medicine. Elsevier, London
Banik BL, Fattahi P, Brown JL (2016) Polymeric nanoparticles: the future of nanomedicine. Wiley interdiscip Rev Nanomed Nanobiotechnol 8:271–299
Elsabahy M, Wooley KL (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev 41:2545–2561
Crisponi G, Nurchi VM, Lachowicz JI (2017) Toxicity of nanoparticles: etiology and mechanisms. In: Grumezescu AM (ed) Antimicrobial Nanoarchitectonics. Elsevier, Bucharest
Avcıbaşı U, Avcıbaşı N, Akalın HA, Ediz M, Demiroğlu H, Gümüşer FG, Özçalışkan E, Türkcan C, Uygun DA, Akgöl S (2013) Synthesis and biodistribution of novel magnetic poly(hema-aph) nanopolymer radiolabeled with iodine-131 and investigation its fate in vivo for cancer therapy. J Nanopart Res 15:2021
Toprak A, Görgün C, Kuru Cİ, Türkcan C, Uygun M, Akgöl S (2015) Boronate affinity nanoparticles for RNA isolation. Mat Sci Eng C Mater Biol Appl 50:251–256
Kuru Cİ, Türkcan C, Uygun M, Okutucu B, Akgöl S (2016) Preparation and characterization of silanized poly (HEMA) nanoparticles for recognition of sugars. Artif Cells Nanomed Biotechnol 44:835–841
Akgöl S, Kaçar Y, Özkara S, Yavuz H, Denizli A, Arıca MY (2001) Immobilization of catalase via adsorption onto l-histidine grafted functional pHEMA based membrane. J Mol Catal B-Enzym 15:197–206
Bakan B, Kayhan CT, Karayildirim CK, Dağdeviren M, Gülcemal S, Yıldırım Y, Akgöl S, Yavaşoğlu NUK (2019) Synthesis, characterization, toxicity and in vivo imaging of lysine graft polymeric nanoparticles. J Polym Res 26:239
Avcıbaşı U, Ateş B, Ünak P, Gümüşer FG, Gülcemal S, Ol KK, Akgöl S, Tekin V (2019) A novel radiolabeled graft polymer: investigation of the radiopharmaceutical potential using albino wistar rats. Appl Radıat Isot. 154:108872
Bangs LB (1989) Uniform latex particles. 41 th National meeting, American Association for Clinical Chemistry, Seragen Diagnostics, Indianapolis
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Maron DM, Ames BN (1983) Revised methods for the salmonella mutagenicity test. Mutat Res 113:173–215
Florence C, Stanley P, Zemke R (1997) Occupational therapy for independent-living older adults: a randomized controlled trial. JAMA 278(16):1321–1326
Bacterial reverse mutation test ASTM‐F756‐00 (2013) Standard practice for assessment of hemolytic properties of materials
Parnham MJ, Wetzig H (1993) Toxicity screening of liposomes. Chem Phys Lipids 64:63–274
Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T (2003) In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomater 24:1121–1131
Xiong Y, Jiang W, Shen Y, Li H, Sun C, Ouahab J, Tu J (2012) A poly (γ, L-glutamic acid)-citric acid based nanoconjugate for cisplatin delivery. Biomater 33:7182–7193
Grabinski C, Hussain S, Lafdi K, Braydich-Stolle L (2007) Effect of particle dimension on biocompatibility of carbon nanomaterials. Carbon 45:2828–2835
Couvreur P, Kante B, Grislain L, Roland M, Speiser P (1982) Toxicity of polyalkylcyanoacrylate nanoparticles II: doxorubicin-loaded nanoparticles. J Pharm Sci 71:790–792
Hussain SM, Hess KL, Gearhart JM, Geiss KT (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro 19:975–983
Voigt N, Henrich-Noack P, Kockentiedt S, Hintz W, Tomas J, Sabel BA (2014) Toxicity of polymeric nanoparticles in vivo and in vitro. J Nanopart Res 16:2379
Monteiro-Riviere NA, Inman AO, Zhang LW (2009) Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol Appl Pharmacol 234:222–235
Türkmen D, Bereli N, Çorman ME, Shaikh H, Akgöl S, Denizli A (2014) Molecular imprinted magnetic nanoparticles for controlled delivery of mitomycin C. Artif Cell Nanomed Biotechnol 42:316–322
Roointan A, Farzanfar J, Mohammadi-Samani S, Behzad-Behbahani A, Farjadian F (2018) Smart pH responsive drug delivery system based on poly (HEMA-co-DMAEMA) nanohydrogel. Int J Pharm 552:301–311
Saraei M, Sarvari R, Massoumi B, Agbolaghi S (2019) Co-delivery of methotrexate and doxorubicin via nanocarriers of star-like poly (DMAEMA-block-HEMA-block-AAc) terpolymers. Polym Int 68:1795–1803
Kumar SSD, Surianarayanan M, Vijayaraghavan R, Mandal AB, Macfarlane DR (2014) Curcumin loaded poly (2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid–In vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur J Pharm Sci 51:34–44
Fröhlich E (2012) The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed 7:5577
Louro H, Bettencourt A, Gonçalves LM (2015) M. Nanotechnology applications for tissue engineering. Nanotechnology Applications for Tissue Engineering. Elsevier, New York
Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE (2008) Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm 5:487–495
Chen LQ, Fang L, Ling J, Ding CZ, Kang B, Huang CZ (2015) Nanotoxicity of silver nanoparticles to red blood cells: size dependent adsorption, uptake, and hemolytic activity. Chem Res Toxicol. 28(3):501–509. https://doi.org/10.1021/tx500479m
Slowing II, Wu CW, Vivero-Escoto JL, Lin VS (2009) Mesoporous silica nanoparticles for reducing hemolytic activity towards mammalian red blood cells. Small 5(1):57–62. https://doi.org/10.1002/smll.200800926
Lin YS, Haynes CL (2010) Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J Am Chem Soc 132:4834–4842
Kim D, El-Shall H, Dennis D, Morey T (2005) Interaction of PLGA nanoparticles with human blood constituents. Coll Surf B Biointerfaces 40:83–91
Kim TH, Nah JW, Cho MH, Park TG, Cho CS (2006) Receptor-mediated gene delivery into antigen presenting cells using mannosylated chitosan/DNA nanoparticles. J Nanosci Nanotechnol 6:2796–2803
Dekie L, Toncheva V, Dubruel P, Schact EH, Barrett L, Seymour LW (2000) Poly-L-glutamic acid derivatives as vectors for gene therapy. J Control Release 65:187–202
Huang X, Teng X, Chen D, Tang F, He J (2010) The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomater 31:438–448
Pliska V, Testa B, Van de Waterbeemd H (1996) Lipophilicity and biological activity. In: Mannhold R, Kubinyi H, Timmerman H (eds) Lipophilicity in Drug Action and Toxiocology. VCH, New York, NY, pp 22–26
Phillips R, Karnofsky DA, Hamilton LD, Nickson JJ (1954) Roentgen therapy of hepatic metastases. Am J Roentgenol 71:826–834
Ingold JA, Reed GB, Kaplan HS (1965) Radiation hepatitis. Am J Roentgenol 93:200–208
Wong Hee Kam S, Huguet F (2010) Normal tissue tolerance to external beam radiation therapy: kidney. Cancer Radiother 14:340–343
Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, Prasad PN (2010) In vivo biodistribution and clearance studies using multimodal ORMOSIL nanoparticles. ACS Nano 4:699–708
İçhedef Ç, Teksöz S, Ünak PE, Medine Eİ, Ertay T, Bekiş R (2012) Preparation and characterization of radiolabeled magnetic nanoparticles as an imaging agent. J Nanopart Res 14:1077
Alexiou C, Bergemann C, Schmid R, Hulin P, Schmidt A, Jurgons R, Arnold W, Parak FG (2002) Enrichment and biodistribution of a magnetically targeted drug carrier. Eur Cell Mater 3:135–137
Kilcar AY, Muftuler FZB, Enginar H, Tekin V, Medine EI, Unak P (2004) Synthesis, characterization and biodistribution of 99mTc-Bioquin-HMPAO (99mTc-BH) as a novel brain imaging agent. J Radioanal Nucl Chem 302:563–573
Liu M, Zheng Y, Avcibasi U, Liu S (2016) Novel 99mTc(III)-azide complexes [99mTc(N3)(CDO)(CDOH)2B-R] (CDOH2 = cyclohexanedione dioxime) as potential radiotracers for heart imaging. Nucl Med Biol. 43:732–741
Demiroğlu H, Topal G, Parlak Y, Gümüşer FG, Türköz EU, Tekin V, Ateş B, Ünak P, Avcıbaşı U (2018) Radiosynthesis and biodistribution of 99mTc- trimethoprim: a novel radiolabeled antibiotic for bacterial infection imaging using experimental animals. Kafkas Univ Vet Fak Derg 24:393–400
Babak S, Morris M, Cao Q (2018) Determination of cis-4-[18F][1]fluoro-L-proline (18F-FP) normal biodistribution in experiments optimized for hepatic uptake using dynamic and static PET/CT imaging. J Nucl Med 59(1):1235–1242
Rao V, Guan B, Mutton LN, Bieberich CJ (2012) Proline-mediated proteasomal degradation of the prostate-specific tumor suppressor NKX31. J Biol Chem. 287(43):36331–36340
Austin-Seymour MM, Chen GT, Castro JR, Saunders WM, Pitluck S, Woodruff KH, Kessler M (1986) Dose volume histogram analysis of liver radiation tolerance. Inr J Radiation Oncology Biol Phys 12(1):31–35
Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, Li XA, Schultheiss TE (2010) Radiation-associated kidney injury. Inr J Radiation Oncology Biol Phys 76(3):108–115
Setchelll BP (1986) The movement of fluids and substances in the testis. Aust. 1. BioI Sci 39:193–207
Acknowledgements
The authors are thankful for the financial support from the Manisa Celal Bayar University Coordination Unit of Scientific Research Projects (BAP) (Project number: 2017-016). We thank to Buket Ateş for the technical assistance during the animal experiments. We also thank Norma R. de Yagcier and Mahdi Rajabimovahed for reviewing our study as native English speakers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avcıbaşı, U., Türkyarar, T., Karadağ, A. et al. Preparation of a 99mTc-labeled graft polymer and its in vitro and in vivo evaluation. J Radioanal Nucl Chem 329, 511–525 (2021). https://doi.org/10.1007/s10967-021-07817-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07817-6