Abstract
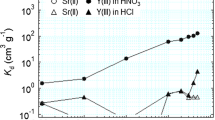
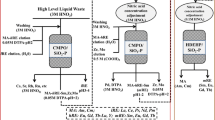
(HDEHP + Hexa)/SiO2-P, a silica-based adsorbent, was prepared and used to separate Y(III) from a mixed solution of Sr(II) and Y(III). The adsorption behavior of the adsorbent was investigated using batch tests. The adsorbent exhibited high adsorption performance for Y(III) in low nitric acid concentrations and weak adsorption performance for Y(III) at higher concentrations. In contrast, Sr(II) was not adsorbed in either acid concentration range. The same tendency was observed under hydrochloric acid conditions. Y(III) separation from a mixed solution of Sr(II) and Y(III) was verified by a column test. Overall, the (HDEHP + Hexa)/SiO2-P adsorbent can separate Y(III) from Sr(II).







Similar content being viewed by others
References
Zhamg A, Kuraoka E, Kumagai M (2007) Development of the chromatographic partitioning of cesium and strontium utilizing two macroporous silica-based calix[4]arene-crown and amide impregnated polymeric composites: PREC partitioning process. J Chromatogr A 1157:85–95
Wu Y, Kim SY, Tozawa D, Ito T, Tada T, Hitomi K, Kuraoka E, Yamazaki H, Ishii K (2012) Equilibrium and kinetic studies of selective adsorption and separation for strontium using DtBuCH18C6 loaded resin. J Nucl Sci Technol 49:320–327
Chakravarty R, Pandey U, Manolkar RB, Dash A, Venkatesh M, Pillai MRA (2008) Development of an electrochemical 90Sr-90Y generator for separation of 90Y suitable for targeted therapy. Nucl Med Biol 35:245–253
Lee JS, Park UJ, Son KJ, Han HS (2009) One column operation for 90Sr/90Y separation by using a functionalized-silica. Appl Radiat Isot 67:1332–1335
Innocenzi V, Michelis ID, Ferella F, Beolchini F, Kopacek B, Vegliò F (2013) Recovery of yttrium from fluorescent powder of cathode ray tube, CRT: Zn removal by sulphide precipitation. Waste Manag 33:2364–2371
Wang Y, Liao W, Li D (2011) A solvent extraction process with mixture of CA12 and Cyanex272 for the preparation of high purity yttrium oxide from rare earth ores. Sep Purif Technol 82:197–201
Tian F, Sun X, Liu X, Zhang H, Liu J, Guo H, Zhang Y, Meng C (2020) Effective adsorptive denitrogenation from model fuels over yttrium ion-exchanged Y zeolite. Chin J Chem Eng 28:414–419
Wanga Y, Huanga C, Li F, Donga Y, Zhaoa Z, Sun X (2016) The development of sustainable yttrium separation process from rare earth enrichments using bifunctional ionic liquid. Sep Purif Technol 162:106–113
Tazoe H, Obata H, Yamagata T, Karube Z, Nagai H, Yamada M (2016) Determination of strontium-90 from direct separation of yttrium-90 by solid phase extraction using DGA Resin for seawater monitoring. Talanta 152:219–227
Xu Y, Kim SY, Ito T, Nakazawa K, Funaki Y, Tada T, Hitomi K, Ishii K (2012) Adsorption and separation behavior of yttrium and strontium in nitric acid solution by extraction chromatography using a macroporous silica-based adsorbent. J Chromatogr A 1263:28–33
Kim SY, Kawamura T, Ito T (2019) Adsorption of Sr(II) and Y(III) by extraction chromatography using DtBuCH18C6-impregnated adsorbent, Global 2019, Seattle, WA, 22–27 Sept 2019
Kawamura T, Ito T, Kim SY (2019) Adsorption and separation behavior of strontium and yttrium using a silica-based CMPO adsorbent. J Radioanal Nucl Chem 320:9–14
Kudo T, Ito T, Kim SY (2017) Adsorption behavior of Sr(II) from high-level liquid waste using crown ether with ionic liquid impregnated silica adsorbent. Energy Procedia 131:189–194
Zhang A, Xiao C, Liu Y, Hu Q, Chen C, Kuraoka E (2009) Preparation of macroporous silica-based crown ether materials for strontium separation. J Porous Mater 17:153–161
Dutta S, Mohapatra PK, Raut DR, Manchanda VK (2011) Chromatographic separation of carrier free 90Y from 90Sr using a diglycolamide based resin for possible pharmaceutical applications. J Chromatogr A 1218:6483–6488
Tkac P, Vandegrift GF, Lumetta GJ, Gelis AV (2012) Study of the interaction between HDEHP and CMPO and its effect on the extraction of selected lanthanides. Ind Eng Chem Res 51:10433–10444
Ho YS, Mckay G (1999) Pseudo-second order model for sorption process. Process Biochem 34:451–465
Lin J, Wang L (2009) Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front Environ Sci Eng 3(3):320–324
Naushad M, Al Othman ZA, Awual MR, Alam MM, Eldesoky GE (2015) Adsorption kinetics, isotherms, and thermodynamic studies for the adsorption of Pb2+ and Hg2+ metal ions from aqueous medium using Ti(IV) iodovanadate cation exchanger. Ionics 21:2237–2245
Wu H, Kim SY, Miwa M, Matsuyama S (2021) Synergistic adsorption behavior of a silica-based adsorbent toward palladium, molybdenum, and zirconium from simulated high-level liquid waste. J Hazard Mater 411:125136
Lima EC, Gomes AA, Tran HN (2020) Comparison of the nonlinear and linear forms of the van’t Hoff equation for calculation of adsorption thermodynamic parameters (∆S° and ∆H°). J Mol Liq 311:113315
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190:916–921
Ueberbacher R, Rodler A, Hahn R, Jungbauer A (2010) Hydrophobic interaction chromatography of proteins: thermodynamic analysis of conformational changes. J Chromatogr A 1217:184–219
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J Appl Chem 3(1):28–45
Zhang A, Hu Q (2010) Adsorption of cesium and some typical coexistent elements onto a modified macroporous silica-based supramolecular recognition material. Chem Eng J 159:58–66
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawamura, T., Wu, H. & Kim, SY. Adsorption and separation behavior of strontium and yttrium using a silica-based bis(2-ethylhexyl) hydrogen phosphate adsorbent. J Radioanal Nucl Chem 329, 1001–1009 (2021). https://doi.org/10.1007/s10967-021-07806-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07806-9