Abstract
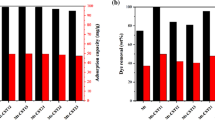
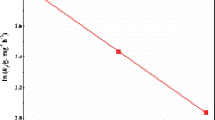
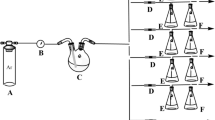
Starch not only has the advantage of inexpensive and biodegradable compared to other adsorbed materials but also contains a large amount of chemically modified hydroxyl groups. Using starch as the raw material, an amino-functionalized starch-based adsorbent was prepared by a three-step reaction of esterification-crosslinking- aminolysis. The samples were characterized by FTIR, EDS, and XPS. The adsorption performance of amino-functionalized starch-based adsorbents for uranyl ions in aqueous solutions was studied, and the adsorption kinetics and adsorption thermodynamics were analyzed. The results show that after the starch-based adsorbent is functionalized with amino groups. Its adsorption performance for uranyl ions is significantly improved. The adsorption behavior of amino-functionalized starch-based adsorbents for uranyl ions conforms to the Langmuir isotherm adsorption model and the quasi-second-order adsorption kinetic equation. The adsorption process mainly depends on the coordination and chelation between uranyl ions and –NH2 on the surface of the adsorbents. And the adsorption process is exothermic. Finally, satisfactory results were obtained under gentle conditions. Under the conditions of pH 6, C0 of 35 mg/g, T of 25 ℃, m of 0.25 g/L, and t of 5 h, the adsorption capacity of the adsorbent for uranyl ions is 118.92 mg/g. This shows that the amino-functionalized starch-based adsorbent has potential application prospects in terms of nuclear energy-containing uranium wastewater.













Similar content being viewed by others
References
Liu Y, Yang PF, Li Q, Liu Y, Yin J (2019) Preparation of FeS@Fe3O4 core-shell magnetic nanoparticles and their application in uranyl ions removal from aqueous solution. J Radioanal Nucl Chem 321(2):499–510
Gulay B, Arica MY (2017) Polyethylenimine and tris (2-aminoethyl) amine modified p (GA-EGMA) microbeads for sorption of uranium ions. Equilibrium kinetic and thermodynamic studies. J Radioanal Nuclear Chem 312(2):293–303
Kusaka R, Watanabe M (2018) Mechanism of phase transfer of uranyl ions: a vibrational sum frequency generation spectroscopy study on solvent extraction in nuclear reprocessing. J Phys Chem Chem Phys 20(47):29588–29590
Mahmoud MR, Othman SH (2018) Efficient decontamination of naturally occurring radionuclide from aqueous carbonate solutions by ion flotation process. J Radiochimica Acta 106(6):465–476
Behrooz MS, Fasihi J, Samadfam M, Sepehrian H, Ashtari P, Mahani M, Arabieh M (2018) Synergistic coupled transport of uranyl ion across bulk liquid membrane mediated by dioxa-diazamacrocycle and oleic acid. J Radioanal Nuclear Chem 316(1):9–16
Zaoui F, Didi MA, Villemin D (2013) Investigation of 7-((dioctylamino) methyl) quinoline-8-ol for uptake and removal of uranyl ions. J Radioanalytical Nuclear Chem 295(1):419–424
Zhao D, Zhang Q, Xuan H, Chen Y, Zhang K, Feng S, Alsaedi A, Hayat T, Chen C (2017) EDTA functionalized Fe3O4/graphene oxide for efficient removal of U (VI) from aqueous solutions. J Colloid Interface Sci 506(1):300–307
Arica MY, Bayramoglu G (2016) Polyaniline coated magnetic carboxymethylcellulose beads for selective removal of uranium ions from aqueous solution. J Radioanal Nucl Chem 310(2):711–724
Erkaya IA, Arica MY, Akbulut A, Bayramoglu G (2014) Biosorption of uranium (IV) by free and entrapped Chlamydomonas reinhardtii: kinetic equilibrium and thermodynamic studies. J Radioanal Nuclear Chem 299(3):1993–2003
Bayramoglu G, Arica MY (2016) MCM-41 silica particles grafted with polyacrylonitrile: modification in to amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous Mesoporous Mater 226:117–124
Mondal H, Karmakar M, Chattopadhyay PK, Singha NR (2019) Starch-g-tetrapolymer hydrogel via in situ attached monomers for removals of Bi (III) and/or Hg (II) and dye (s): RSM-based optimization. J Carbohydr Polym 213(2):428–440
Dong A, Xie J, Wang W, Yu L, Liu Q, Yin Y (2010) A novel method for amino starch preparation and its adsorption for Cu (II) and Cr (VI). J hazardous Mater 181(1–3):448–454
Wang G, Zhang F, Liang (2017) Preparation and characterization of cationic starch dye adsorption materials. J Chem Ind Eng 68(5):2112–2121
Zhang F, Wang G, Liang (2017) Preparation of cross-linked carboxymethyl starch and its adsorption performance for heavy metal ions. J Chem Ind Eng Progr 36(07):2554–2561
Chen Y, Zhao W, Wang H, Meng X, Zhang L (2018) A novel polyamine-type starch/glycidyl methacrylate copolymer for adsorption of Pb (II), Cu (II), Cd (II) and Cr (III) ions from aqueous solutions. J R Soc Open Sci 5(6):180281
Zhou L, Ouyang J, Liu Z, Huang G, Wang Y, Li Z, Adesina AA (2019) Highly efficient sorption of U (VI) from aqueous solution using amino/amine-functionalized magnetic mesoporous silica nanospheres. J Radioanal Nuclear Chem 319(3):987–995
Zeng Y, Lei J, Li Qi (2019) Preparation of ZnS / AC composite and its adsorption properties for uranyl ions. J Fine Chem 36(4):751–758
Kim MY, Seo H, Lee TG (2020) Removal of Hg (II) ions from aqueous solution by poly (allylamine-co-methacrylamide-co-dimethylthiourea). J Ind Eng Chem 84(2):82–86
An FQ, Wu RY, Li M, Hu TP, Gao JF, Yuan ZG (2017) Adsorption of heavy metal ions by iminodiacetic acid functionalized D301 resin: kinetics, isotherms and thermodynamics. J Reactive Funct Polym 118(2):42–50
Sprynskyy M, Kovalchuk I, Buszewski B (2010) The separation of uranium ions by natural and modified diatomite from aqueous solution. J Hazardous Mater 181(1–3):700–707
Acknowledgement
This study was financially supported by the Innovation and Entrepreneurship Training Program for College Students in Hunan Province (S201910555093), and the Innovation and Entrepreneurship Training Program of the University of South China (X2019067, X2019069)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, Z., Liu, C., Zhang, B. et al. Preparation of amino-functionalized starch-based adsorbent and its adsorption behavior for uranyl ions. J Radioanal Nucl Chem 328, 1253–1263 (2021). https://doi.org/10.1007/s10967-021-07733-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07733-9