Abstract
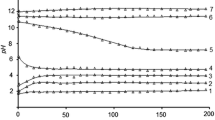
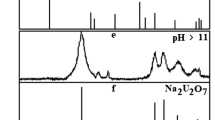
The chemical stability of uranyl arsenates of rare-earth elements with the general formula MIII(AsUO6)3⋅16H2O (MIII–La–Lu) in aqueous solutions has been studied in a wide range of acidity. The acid–base ranges of the existence of these compounds in aqueous solutions were established, the transformation products formed outside these ranges were identified, and the solubility of MIII(AsUO6)3⋅16H2O was determined. Based on the experimental data obtained, the solubility products, Gibbs free energies of the formation of the rare-earth elements uranyl arsenates were calculated, the solubility curves of the studied compounds were computed, and the speciation diagrams of uranium(VI), arsenic(V), and rare-earth elements in saturated aqueous solutions and equilibrium solid phases were constructed.



Similar content being viewed by others
References
Locock AJ, Burns PC, Duke MJM, Flynn TM (2004) Structures and synthesis of layered and framework amine-bearing uranyl arsenates and phosphates. Can Mineral 177(8):2675–2684. https://doi.org/10.1016/j.jssc.2004.03.045
Locock AJ, Burns PC (2003) Crystal structures and synthesis of the copper-dominant members of the autunite and meta-autunite groups: torbernite, zeunerite, metatorbernite and metazeunerite. Can Mineral 41:489–502. https://doi.org/10.2113/gscanmin.41.2.489
Locock AJ, Burns PC (2003) Structures and synthesis of framework Rb and Cs uranyl arsenates and their relationships with their phosphate analogues. J Solid State Chem 175(2):372–379. https://doi.org/10.1016/S0022-4596(03)00383-9
Chernorukov NG, Karyakin NV, Suleimanov EV, Chernorukov GN (1994) Synthesis and study of MIAsUO6⋅nH2O compounds. Russ J Inorg Chem 1:23–26
Chernorukov NG, Karyakin NV, Suleimanov EV, Belova YS (1998) Synthesis and study of Mg(PUO6)2⋅nH2O and Mg(AsUO6)2⋅nH2O compounds. Russ J Inorg Chem 3:380–383
Chernorukov NG, Karyakin NV, Suleimanov EV, Belova YS (1997) Synthesis and study of Ba(PUO6)2⋅nH2O and Ba(AsUO6)2⋅nH2O compounds. Russ J Inorg Chem 5:693–697
Chernorukov NG, Karyakin NV, Suleimanov EV, Belova YS (1996) Synthesis and study of Sr(PUO6)2⋅nH2O and Sr(AsUO6)2⋅nH2O compounds. Radiochemistry 5:729–732
Chernorukov NG, Suleimanov EV, Jabarova ST (1998) Synthesis and study of Ni(PUO6)2⋅nH2O and Ni(AsUO6)2⋅nH2O compounds. Russ J Inorg Chem 7:1090–1095
Chernorukov NG, Suleimanov EV, Jabarova ST (1999) Synthesis and study of Co(PUO6)2⋅nH2O and Co(AsUO6)2⋅nH2O compounds. Russ J Inorg Chem 5:782–787
Chernorukov NG, Suleimanov EV, Jabarova ST, Barch SV (2000) Synthesis and study of AII(BVUO6)2⋅nH2O (AII—Mn, Fe Co, Ni, Cu; BV—P, As) compounds. Radiochemistry 1:15–32
Vochten R, De Grave E, Pelsmaekers J (1986) Synthesis, crystallographic and spectroscopic data, solubility, and electrokinetic properties of metakahlerite and its Mn analogue. Am Mineral 71(7–8):1037–1044
Vochten R, Piret P, Goeminne A (1981) Synthesis, crystallographic data, solubility and electrokinetic properties of copper-, nickel- and cobalt˗uranylphosphate. Bull Minéral 104:457–467. https://doi.org/10.3406/bulmi.1981.7496
Vochten R, Goeminne A (1984) Synthesis, crystallographic data, solubility and electrokinetic properties of meta-zeunerite, meta-kirchheimerite and nickel-uranylarsenate. Phys Chem Miner 11:95–100. https://doi.org/10.1007/BF00308011
Chernorukov NG, Suleimanov EV, Barch SV, Alekseev EV (2001) Synthesis and study of lanthanides and yttrium uranyl arsenates. Radiochemistry 1:9–16
Suleimanov EV, Chernorukov NG, Golubev AV (2001) Synthesis, structure and physico-chemical properties of Pb(BVUO6)2⋅nH2O (BV—P, As, V) compounds. Radiochemistry 5:412–417
Chernorukov NG, Nipruk OV, Pykhova YP (2010) On the role of interlayer atoms and molecular water in formation of crystalline structure of salts of uranyl arsenic acid HAsUO6⋅4H2O. Russ J Inorg Chem 55(2):190–194. https://doi.org/10.1134/S0036023610020099
Chukhlantsev VG, Sharova AK (1956) Solubility products of uranyl arsenates. Russ J Inorg Chem 1:36–42
Nipruk OV, Chernorukov NG, Elipasheva EV, Klinshova KA, Bakhmetev MO (2020) State of uranyl arsenates MIAsUO6·nH2O (MI–H+, Li+, Na+, K+, Rb+, Cs+, NH4+) in aqueous solution. J Radioanal Nucl Chem 324(1):233–244. https://doi.org/10.1007/s10967-020-07062-3
Zhiltsova IG, Polupanova LI, Shamriovich EM, Perlina SA (1987) Physico-chemical conditions of formation of ore uranyl arsenate mineralization. Lithol Miner 3:44–54
Chernorukov NG, Nipruk OV, Pykhova YP, Godovanova NS (2012) Study of the state of uranoarsenates MII(AsUO6)2⋅nH2O (MII = Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+) in aqueous solutions. Russ J Gen Chem 82(8):1348–1356. https://doi.org/10.1134/S107036321208004X
Baranovskaya NV, Ageeva EV, Soktoev BR, Narkovich DV, Denisova OA, Matkovskaya TV (2020) Rare earth and radioactive (Th, U) elements in the components of the environment on the territory of Tomsk region. Bull Tomsk Polytech Univ 331(2):17–28. https://doi.org/10.18799/24131830/2020/2/2477
Gob S, Guhring JE, Bau M, Markl G (2013) Remobilization of U and REE and the formation of secondary minerals in oxidized U deposits. Am Mineral 98(4):530–548. https://doi.org/10.2138/am.2013.4275
Zhang W, Chen WT, Gao JF, Chen HK, Li JH (2019) Two episodes of REE mineralization in the Qinling Orogenic Belt, Central China: in-situ U-Th-Pb dating of bastnasite and monazite. Miner Deposita 54(8):1265–1280. https://doi.org/10.1007/s00126-019-00875-7
Nemodruk AA (1976) Analytical chemistry of arsenic. Science, Moscow
Savvin SB (1964) Analytical Applications of Arsenazo III. Part II: determination of thorium, uranium, protactinium, neptunium, hafnium and scandium. Talanta. https://doi.org/10.1016/0039-9140(64)80003-5
Ryabchikov DI, Ryabukhin VA (1966) Analytical chemistry of rare earth elements & yttrium. Science, Moskow
Guillaumont R, Fanghänel T, Fuger J et al (2003) Update on the chemical thermodynamics of uranium, neptunium, and plutonium. Elsevier, Amsterdam
Yungman VS (ed), Glushko VP, Medvedev VA, Gurvich LV (1999) Thermal constants of substances. Wiley, USA
Grenthe I, Fuger J, Koning R et al (2004) Chemical thermodynamics of uranium. North-Holland, Amsterdam
Korostelev PP (1964) Preparation of solutions for chemical and analytical works. Science, Moscow
Kiseleva EK, Suslennikova VM (1959) Reference guide for the preparation of titrated solutions and determining their titres. Typolithography LKVVIA named after A.F. Mozhaisky, Leningrad
Tang M, Holliday KS, Jiang C, Valdez JA, Uberuaga BP, Dickerson PO, Dickerson RM, Wang Y, Czerwinski KR, Sickafus KE (2010) Order-to-disorder phase transformation in ion irradiated uranium—bearing delta—phase oxides RE6U1O12 (RE=Y, Gd, Ho, Yb, and Lu). J Solid State Chem. https://doi.org/10.1016/j.jssc.2010.01.020
Venkata Krishnan R, Jena H, Govindan Kutty KV, Nagarajan K (2010) Heat capacity and thermal expansion coefficient of rare earth uranates RE6UO12 (RE–Nd, Gd and Eu). J Therm Anal Calorim. https://doi.org/10.1007/s10973-009-0618-y
Krishnan RV, Babu R, Panneerselvam G, Ananthasivan K, Antony MP, Nagarajan K (2012) Thermophysical properties of Dy6UO12. Ceram Int. https://doi.org/10.1016/j.ceramint.2012.02.044
Shukla B, Sanjay Kumar NR, Sekar M, Chandra Shekar NV, Jena H, Asuvathraman R (2016) Stability of Dy6UO12 under high pressure and high temperature. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2016.02.202
Nipruk OV, Knyazev AV, Chernorukov GN, Pykhova YP (2011) Synthesis and study of hydrated uranium(VI) oxides, UO3⋅nH2O. Radiochemistry. https://doi.org/10.1134/S1066362211020044
Finch RJ, Hawthorne FC, Miller ML, Ewing RC (1997) Distinguishing among schoepite, [(UO2)8O2(OH)12](H2O)12, and related minerals by X-ray powder diffraction. Powder Diffr 12(4):230–238. https://doi.org/10.1017/S0885715600009799
Chernorukov NG, Nipruk OV, Arova MI, Blazhenova DV (2013) Synthesis and study of polyuranates MIIIU3O10.5⋅6H2O (MIII = La, Ce, Pr, Nd, Sm). Russ J Gen Chem 83(4):642–645. https://doi.org/10.1134/S1070363213040051
Chernorukov NG, Nipruk OV, Arova MI, Chaplieva KA (2014) Preparation and properties of the polyuranates MIIIU2O7.5 (MIII = Y, Tb, Dy, Ho, Er, Tm, Yb, or Lu). Russ J Gen Chem 84(1):6–8. https://doi.org/10.1134/S1070363214010022
Nipruk OV, Chernorukov NG, Chaplieva KA (2017) Synthesis and study of hexauranates MIII[(UO2)6O4.5(OH)6]⋅7H2O (MIII—Nd, Sm, Eu, Gd, Dy). J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-017-5462-0
Zhang Y, Aughterso R, Karatchevtseva I, Kong L, Tran TT, Čejka J, Aharonovich I, Lumpkin GR (2018) Uranyl oxide hydrate phases with heavy lanthanide ions: [Ln(UO2)2O3(OH)]⋅0.5H2O (Ln = Tb, Dy, Ho and Yb). New J Chem 42(15):12386–12393. https://doi.org/10.1039/c8nj01376d
Zhang YJ, Aughterson RD, Zhang ZM, Wei T, Lu K, Cejka J, Karatchevtseva I (2019) Syntheses, crystal structures, and spectroscopic studies of uranyl oxide hydrate phases with La(III)/Nd(III) ions. Inorg Chem 58(16):10812–10821. https://doi.org/10.1021/acs.inorgchem.9b01102
Lu KT, Zhang YJ, Wei T, Cejka J, Zheng RK (2020) Layer-structured uranyl-oxide hydroxy-hydrates with Pr(III) and Tb(III) ions: hydroxyl to oxo transition driven by interlayer cations. Dalt Trans 49(18):5832–5841. https://doi.org/10.1039/d0dt00526f
Kovba LM (1972) Crystal structure of sodium diuranate. Radiochemistry 14:727–730
Nipruk OV, Chernorukov NG, Zakharycheva NS, Kostrova EL (2017) State of rare earth elements uranyl germanates in aqueous solutions. J Radioanal Nucl Chem 311(1):519–529. https://doi.org/10.1007/s10967-016-5044-6
Nipruk OV, Chernorukov NG, Godovanova NS, Kostrova EL (2013) Behavior of uranosilicates MIII(HSiUO6)3·10H2O (MIII = La–Lu, Y) in aqueous solutions. Radiochemistry 55(1):63–71. https://doi.org/10.1134/S1066362213010128
Nipruk OV, Chernorukov NG, Eremina AA, Kostrova EL, Chaplieva KA (2014) Behavior of rare earth uranovanadates in aqueous solutions. Radiochemistry 56(4):392–399. https://doi.org/10.1134/S106636221404006!
Acknowledgements
The work was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation, Project No. 0729-2020-0039.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nipruk, O.V., Chernorukov, N.G., Klinshova, K.A. et al. Chemical stability of rare-earth elements’ uranyl arsenates with general formula MIII(AsUO6)3·16H2O (MIII–La–Lu) in aqueous solution. J Radioanal Nucl Chem 328, 739–751 (2021). https://doi.org/10.1007/s10967-021-07692-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07692-1