Abstract
We report the phenomenon whereby the electron spin resonance signal of human nails intensify after irradiation. Forty nail samples from three donors were divided into three groups (X-ray irradiated, γ-ray irradiated, and control). The extent of signal intensified after irradiation was proportional to the given dose under 11% humidity at 20 °C. This result seemed to contradict previous studies, reporting signal fading. Based on literature and our experimental results, we constructed a model taking both the decay and increase into consideration. Our investigation revealed that rapid deterioration in the signal intensity obscured the increase at humidity levels exceeding 40%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electron spin resonance (ESR) has been widely used for basic research and various applications because it can be used to non-destructively detect unpaired electrons in molecules and radicals that exist in samples. Retrospective dosimetry in radiation disasters and accidents is a typical application; for example, atomic bombs [1, 2], accidents involving nuclear reactors such as the Chernobyl disaster [3], accidents at nuclear test sites [4], and medical accidents [5].
In the context of retrospective dosimetry, the signal characteristics of many biomaterials, such as teeth, bones, nails, belongings, and everything on-site, have been investigated to find adequate samples that would enable the exposed dose of victims and the environment to be reconstructed [6, 7].
A well-researched sample is tooth enamel, for which dose reconstruction methods have been established at doses of 500 mGy and above. However, the current method, which requires a sample to be harvested from a living person, is extremely invasive.
In terms of sample collection, nails are ideal because of the low invasiveness and ease of use of this method. However, previous studies suggested that radiation-induced signals (RISs) decay quickly [8,9,10] as a result of moisture. To stabilize the RIS, storage in a vacuum desiccator or freezer at less than − 20 °C was suggested [11, 12]. The attention of researchers has since shifted to other important issues, especially the separation of signals from noise because mechanical stress also generates a mechanically induced signal (MIS), and the background signal (BKS) exists natively [8, 13, 14]. These signals would have to be removed to enable the radiation dose to be reconstructed from the RIS. The behavior of the RIS under ambient conditions with less humidity has not been studied adequately owing to the shift of researchers’ attention to the separation and stabilization of the RIS.
In an ideal laboratory situation, samples can be stored in vacuum desiccators or freezers at less than − 20 °C. However, in on-site accidents or during transportation, maintaining ideal conditions can be difficult because of the severe circumstances outside. To reduce the humidity, nail samples can be stored with silica gel in an airtight container. During our investigations of the signal behavior under these conditions, we observed that the RIS has an increasing component despite its well-known tendency to fade.
In this work, we validate the existence of the increasing component in the RIS. In this paper, we describe the experimental details and the proposed model.
Theory
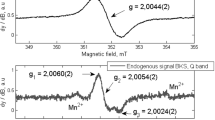
An ESR signal results from the absorption of microwaves by the sample accompanied by the energy transition of the splitting spin states of unpaired electrons under the Zeeman effect. The ESR spectrum is the first derivative waveform of the absorption, and the signal size is defined as the peak-to-peak amplitude of the spectrum normalized by the sample weight, as shown in Fig. 1. Radiation-exposed human nail samples contain unpaired electrons derived from radicals generated by radiation and mechanical stimuli.
To verify the existence of an increasing component in the RIS, an attempt was made to observe the linear relationship between the increments and the given dose or the initial intensity of the RIS that correlates with a given dose. In the latter relationship, the impact of the fluctuation in the sensitivity of an individual sample to radiation can be removed. To distinguish only the increment resulting from the RIS, we also monitored the signal behavior of non-irradiated samples as controls.
Resolving the conflict with previous knowledge necessitated us to establish a model that would explain both our data and existing data comprehensively. Previous studies that reported RIS fading were reviewed, and the humidity conditions and decay information were obtained from them.
Based on the literature review, we constructed a model that includes fading as a function of humidity as well as the newly discovered phenomenon of increased signal intensity. This model was fitted to the present data, and the relationship between the size of the RIS increment and the initial RIS was estimated.
Materials and methods
Nail clippers were used to collect 40 nail samples from the fingers and toes of three donors who were in their thirties, forties, and fifties after obtaining their consent. These samples were separated into three groups: X-ray irradiation, γ-ray irradiation, and control. First, all signals were erased by soaking the samples in water for 12 h and drying them for four days. After drying, the background signals were monitored for a few days, following which the samples were divided into two groups and irradiated accordingly. The energy of the X-rays was less than 160 keV, with a typical energy of 60 keV. The γ-ray source was cesium-137. All samples were stored at 20 °C and 11% humidity and monitored for more than one month.
The ESR measurements were conducted on a JES-FA100 instrument (JEOL Ltd., Japan) with an X-band microwave generator (9.4 GHz). The measurement settings were as follows: microwave power, 1 mW; scanning field, 328.5 - 343.5 mT; time constant, 0.3 s; sweep time 30 s; and modulation width, 1 mT. The ESR signal intensity was defined as the peak-to-peak amplitude of the averaging spectrum with samples rotated horizontally every 45° after normalization by sample weight. An uncertainty of 4% was applied because the main component of the measurement error was expected to be the uncertainty in the vertical position of the sample, and a 1-mm vertical shift of the sample resulted in a 4% change.
Twelve samples were subjected to X-ray irradiation using a commercial device (Cabinet X-ray system model CP-100, Fraxitron Ltd., USA). In this study, the tube voltage and current were 160 kV and 6.3 mA, respectively. No filter was applied. The most frequent energy of the photons was approximately 60 keV. The dose rate was 1.2 ± 0.04 Gy/min. Fractionated irradiation was performed to check the linear response, and the total doses of the 12 samples were 23, 47, and 92 Gy for each group of four samples, respectively.
A Gammacell 40 Exactor Low Dose Rate Research Irradiator (Best Theratronics Ltd., Ottawa, Canada) was used to irradiate the 12 samples with gamma rays. In this irradiator, the sample canister (10 cm high and 31.2 cm in diameter) was located between two cesium-137 sources. The dose rate was 0.79 Gy/min. The total doses the samples received were 24, 48, and 97 Gy for each group of four samples, respectively.
To measure the air-absorbed dose in the radiation fields where the samples were located, we used glass dosimeters (Dose Ace GD-352 M) for X-rays and GD-302 M (Chiyoda Technol Corporation, Tokyo, Japan) for gamma rays.
During the monitoring period, all samples were stored in airtight plastic bags with silica gel (Medium Granular, Blue, FUJIFILM Wako Pure Chemical Corporation, Japan) to maintain the low humidity level. These plastic bags were kept in the dark box at 20 °C. The humidity and temperature were monitored throughout the monitoring period using a logger (LR5001, Hioki, Japan).
Analysis of Signal Changes
We conducted a literature search and found three reports that describe RIS fading, as detailed in Table 1. Two of these three reports reported the humidity among the experimental conditions.
In one literature [8], when the samples were stored in a vacuum oven, it was not possible to observe the decay for five days. However, under 60% humidity, a decline to the background level was observed within five days. Soaking the samples in water, the half-life of RIS was approximately 5 min. The fading in water was reproduced in another study [10].
Based on the first study [8] listed in the table, the fading upon soaking in water follows exponential decay, as shown in Eq. (1).
where I(t) is the signal intensity at time t, which is the number of days after irradiation. The fading was characterized by three factors: the ratio of decay component A, the ratio of constant component Iconst, and the half-life in units of days thalf. The summation of the first and second factors was almost one.
The peak-to-peak amplitude of the spectra was measured after 1 h, 1 day, and 5 days at 60% humidity and under vacuum [8]. This information was substituted into Eq. (1) to calculate the factors at 60% humidity and under vacuum. The results are presented in Table 2. In our experiment, the humidity was maintained at approximately 11% for the entire storage period. The three factors of Eq. (1) at 11% humidity were estimated from values in Table 2 by determining the tendency using the polynomial of humidity. The humidity in the vacuum oven was assumed to be 0%, and that in the water was 100%. As a result, A was 0.29, Iconst was 0.75, and thalf was 0.89.
To consider the phenomenon of increasing signal intensity, a typical saturation curve was used. In addition, based on the analysis of the non-irradiated samples, the term for BKS behavior was added. The model for the experimental data is shown in Eq. (2).
where I0 is the initial BKS size, ABKS is the increase or decrease in the rate of the BKS, IRIS is the initial RIS, aRIS is the final increment of the RIS, and tir is the time of irradiation. Moreover, I0+ ABKSt expresses the BKS at time t, aRIS(t − tir)/√(1 + (t − tir)2) describes the increasing behavior of the RIS, and Aexp(− t/thalf) + Iconst is the decay factor for the RIS.
Equation (2) was fitted to the data with the three decay parameters for 11% humidity.
Results and discussion
The BKS intensity immediately after pretreatment for eliminating signals before this experiment was 0.061/mg on average, and its standard error was 0.005/mg for all samples. The signal intensity is shown in arbitrary units. The breakdown was as follows: from the control group: (0.052 ± 0.005)/mg; from the group irradiated by X-rays: (0.089 ± 0.009)/mg, and from the group irradiated by gamma rays: (0.054 ± 0.003) /mg.
The intensity of the BKS was measured before irradiation. It was possible to observe a slight increase in BKS: the rate of increase was (0.0008 ± 0.0002)/mg/day, (0.0017 ± 0.0003)/mg/day, and (0.0019 ± 0.0004)/mg/day for the control samples and those irradiated by X-rays and gamma rays, respectively. The ratios of increase relative to the initial BKS intensity were 1.5%/day, 1.9%/day, and 3.7%/day for the control, X-ray, and gamma-ray groups, respectively.
The sensitivities for X-rays and gamma rays were similar. Additionally, the inter-donor variety of sensitivity was not found. The average sensitivity from all samples was (0.0046 ± 0.0001)/Gy and (0.0046 ± 0.0002)/Gy for X-rays and (0.0046 ± 0.0002)/Gy for gamma rays. According to the Kruskal–Wallis test, the null hypothesis that all data from different donors belong to the same mother distribution was not denied.
Figure 2 shows the typical behavior of the signal intensity from a sample irradiated by X-rays, which the signal additionally increased for the first few days, after which it maintained to increase derived from the BKS.
Typical behavior of signal intensity. This sample was a fingernail from a male donor in his thirties and was irradiated by 92-Gy X-rays. The black points represent the data before irradiation, and the red points show those after irradiation. The gray lines represent the results of monitoring the control samples. The fluctuation in the error bars is the result of sample positioning during ESR measurements. The size of the fluctuation was 4% for a 1-mm shift of the sample position
The aRIS obtained by fitting Eq. (2) to the data after irradiation denotes the maximum size of the additional increment without fading. The value of aRIS was correlated to the given dose for both the X-ray and gamma-ray groups. The tendency was more explicit in the X-ray group than the gammma-ray group, with a correlation coefficient of 0.94. For the gamma-ray group, it was 0.38. When both of these sets of data were merged, the correlation coefficient was 0.63. The average aRIS/Gy for all samples was 0.0036, which was 78% of the average sensitivity.
Figure 3 shows the relationship between aRIS and the initial RIS intensity IRIS. This figure implies that the excess from the initial RIS generated by X-rays would have been 107% if fading did not occur. In reality, the convolution of both the increasing and fading intensity determines the maximum size of the increment. Taking this into account, the full excess of the RIS was approximately 50% of the initial RIS at 11% humidity. Some data of the gamma-ray group were mostly consistent with those of the X-ray group. The reason for specific data not being correlated with the initial RIS remains unknown, but it is worthwhile mentioning that these noncorrelated data were collected from one donor–a male in his fifties.
The substitution of the aRIS values at irradiation of 50 Gy into Eq. (2) made it possible to calculate the time dependence of the signal intensity under various humidity conditions, as shown in Fig. 4.
Calculated intensity for a constant value of aRIS (50 Gy) at different humidity levels using Eq. (2)
At humidity levels in excess of 40%, the fading was dominant; therefore, we can suppose that, at these humidity levels, the increase we observed remained undetected in previous studies. However, even if the humidity was less than 20%, the excess resulting from the increase may not have been apparent at a low dose. The inaccuracy of the measurement is a few percent of the total signal size of the RIS, MIS, and BKS. This result might explain why the RIS from human nails is well known to undergo fading.
Conclusions
Under relatively dry conditions, such as humidity levels below 11%, the radiation-induced ESR signal measured from human nails continued increasing for several days after irradiation. The initial response to X-rays and gamma rays was similar for all donors; however, the sample set from one of the donors showed a distinct tendency to differ from others toward the phenomenon of signal increment. Further study is required, including the extent to which the signal intensifies depending on the particular donor.
References
Ikeya M (1984) Japanese J Rad Phys. https://doi.org/10.1143/JJAP.23.L697
Hirai Y (2019) Tooth enamel ESR doses and cytogenetic doses of Nagasaki atomic-bomb survivors in comparison with DS02R1 doses. Int J Radiat Biol 95:321–328
Ishii H (1990) J Nucl Sci Tech. https://doi.org/10.3327/jnst.27.1153
Zhumadilov Z (2012) IPSHU English Res Rep Ser. https://doi.org/10.15027/33634
Trompier F (2010) Health Phys. https://doi.org/10.1097/01.HP.0000346334.78268.31
Nakajima T (1982) Int J Appl Radiat Isot. https://doi.org/10.1016/0020-708x(82)90236-8
Dalgarno B (1989) Int J Appl Radiat Isot. https://doi.org/10.1016/0883-2889(89)90034-8
Sholom S (2016) J Radiat Med. https://doi.org/10.1016/j.radmeas.2016.02.014
Symons MCR (1995) Radiat Prot Dosim. https://doi.org/10.1093/oxfordjournals.rpd.a082591
Sholom S (2017) Radiat Phys Chem. https://doi.org/10.1016/j.radphyschem.2017.06.009
Reyes R (2012) Health Phys. https://doi.org/10.1097/HP.0b013e31824ac338
Reyes R (2008) Radiat Environ Biophys. https://doi.org/10.1007/s00411-008-0178-8
Romanyukha A (2007) Radiat Meas. https://doi.org/10.1016/j.radmeas.2007.05.026
Sholom S (2019) Radiat Prot Dosim. https://doi.org/10.1093/rpd/ncz019/5365232
Acknowledgements
This work was supported by the Program of the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science and MEXT/JSPS KAKENHI Grant Number 18KK0147. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This experiment was approved by the ethics committee of Hiroshima University (No. E-2843).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hirota, S., Gonzales, C.A.B., Yasuda, H. et al. Electron spin resonance signal of human nails: increase after irradiation. J Radioanal Nucl Chem 328, 1369–1373 (2021). https://doi.org/10.1007/s10967-020-07540-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07540-8