Abstract
The aim of this study is the development of a separation technique for geological samples, namely bismuthinites, with the goal to isolate Tl from excess amounts of Bi. This will enable an isotopic measurement of the Tl content on the ultra-trace level to identify a possible signature of Bi α-decay. The separation of Tl from other elements like e.g. Pb has been the subject of numerous studies. However, the separation of ultra-traces of Tl from bulk Bi had not yet been investigated sufficiently. This paper describes a separation technique for the system Bi/Tl for application to ultra-trace analysis. The separation technique uses a column chromatographic system, utilizing the specific redox behavior of Tl. The results show, that Tl can be successfully separated in appearance of vast excess amounts of Bi, showing recovery rates up to 96%. The developed procedure was successfully applied for the ultra-trace analysis on a number of bimuthinites using ICP-MS. On base of the results it is discussed, which prerequisites bimuthinite samples must fulfill to be suitable for the quantification of Bi alpha decay on base of the Tl isotopic ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
First proof of the α-decay of 209Bi
In 2003 de Marcillac et al. detected decay events during background measurements with a scintillation bolometer using bismuth germanate (BGO) detectors [1]. Scintillation bolometers are commonly used to discriminate γ-radiation from α-radiation. The detected events yielded evidence for α-particles with an energy of 3.130 ± 0.016 MeV. In total, 128 decay-events were detected with a measuring time of 432,000 s using a 92 g BGO crystal [1]. These results were used to calculate a half life value of 1.9 ± 0.2 × 1019 a for 209Bi. This is in agreement with the expected α decay of 209Bi to 205Tl based on the mass difference of these nuclei. However, no further investigations regarding the stable decay-product 205Tl were performed back then. A direct detection of the decay product 205Tl is very challening, because BGO is an artificial material and therefore it cannot be expected that the number of 205Tl atoms generated since the production of the BGO crystal is sufficient to be detected e.g. by mass spectrometry.
Ambition for the direct proof of the α-decay of 209Bi
However, if (geologically) old Bi containing materials are used, it can be expected that the number of generated 205Tl atoms is sufficient for detection by ICP-MS. Based on the half life, in 1 g of Bi during 1 Ma, about 108 atoms of 205Tl are generated, an amount, that is well accessible to measurements by MC-ICP-MS (multi collector inductively coupled plasma mass spectrometry). If there are already Tl traces present in the material—what cannot be excluded—205Tl from Bi decay will shift the 205Tl/203Tl ratio. Therefore, our strategy is to proof the α-decay of 209Bi directly by isotopic deviations of the Tl content. This method has the potential for a high accuracy using old Bi materials. For this goal several previous working steps are needed, (1) a suitable separation technique and (2) suitable raw materials, namely geologically old Bi minerals with low Tl content.
A specific separation technique for the system Bi/Tl on ultra-trace level
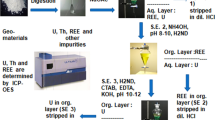
In 1999, Rehkämper and Halliday published their studies concerning a separation procedure, based upon the combination of previously developed separation techniques for the system Pb/Tl, for the purpose of isotope analysis of geological samples by MC-ICP-MS [2]. They used a two-stage anion exchange chromatography to separate trace amounts of Tl from other elements of the geological matrix. The separation uses the specific redox behaviour of Tl (see below). This technique has been successfully applied to isotopic analysis of Tl in Pb minerals [2], e.g. isotopic analyses of Tl in seawater and deposits [3] as well as in meteorites [4]. The procedure makes use of the distinct redox behavior of Tl: Tl(III) is not thermodynamically stable in aqueous solutions, but it can be stabilized under oxidative conditions. For oxidation, a mixture of HBr–HNO3–Br2 (aq) has been used. Under this conditions, Tl forms an anionic complex [TlBr4]−, which can be separated from the Bi-species using anion exchange chromatography, while Pb is expected to run through the column as a cationic species. During the separation, the column has to be rinsed continuously with the oxidizing agent to keep constant oxidative conditions, otherwise the Tl(III) complex gets partially reduced to Tl+(aq) which is eluted prematurely. After successful separation of the Pb content, in a second step, the Tl complex is reduced with SO2 to Tl+ for elution [2]. The advantage of this method is the introduction of all redox agents in gaseous form, which can be expected to suppress the introduction as a contaminant on an ultra trace level. This technique has been successfully applied to ultra-trace analysis of Tl in Pb-minerals and was adapted in this work onto the systen Bi/Tl. First pre-tests of the Bi/Tl separation chemistry were performed during the diploma thesis of Beu [5].
Suitable raw materials
The second step needed for our purpose would be the acquisition of suitable Bi raw materials, preferably untreated bismuthinites, since these minerals often contain comparatively less amounts of natural Tl impurities. Although metal sulphides also might contain Tl traces, the literature lists examples of sulphidic minerals with very low known Tl concentrations, e.g. pyrite (FeS2; < DL (10 ppb)), sphalerite (ZnS; < 50 ppb) and galena (PbS; < 50 ppb) [6, 7]. This made us confident that it is possible to find also bismuthinites (Bi2S3) with such low Tl impurities. A high geological age of the samples is nevertheless essential, otherwise an isotopic shift to 205Tl would not be recognizable. The precise criteria for the selection of suitable raw materials will be discussed later in the experimental section. The measurement of the Tl content and the small expected isotopic shift to 205Tl is technically possible using MC-ICP-MS. With these results the estimation of the half life of 209Bi is possibly more accurate than using low numbers of decay-events for a calculation. Additonally, in the case of success, the development of a geological chronometer for Bi-material would be possible.
Problems to be dealt within this study
The main goal of this study is the transfer of the separation technique of Rehkämper et al. on the system Bi/Tl. For proof of principle, the first approaches of the separation technique were performed using n.c.a. (non carrier added) amounts of 204Tl as radioactive tracer. The subsequent analysis was performed using liquid scintillation counting (LSC). Further, Bi minerals in sulphidic form were acquired and first screened for their natural Tl content using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Finally, the developed separation technique and individual modifications were performed using bismuthinite samples with subsequent analysis using ICP-MS. Tables 1 and 2 in the supplementary information give an overview about the selected bismuthinite samples, their Tl contents, their crystal sizes and their corresponding deposits.
Experimental
Development of a separation procedure for the system Bi/Tl and proof of principle using 204Tl as a radioactive tracer
All solutions were prepared freshly at the day of use. Details of the used chemicals are given in Table 1. Br2 water was prepared using 3 mL Br2 and 6 mL water. The oxidative solution 1 was prepared mixing 9 M HBr, 0.5 M HNO3 and Br2(aq) in the ratio 1:1:0.01. The reductive solution 2 was prepared bubbling SO2 through 0.1 M HCl. Aqua regia was prepared mixing 3 parts of 37% HCl with 1 part of 65% HNO3.
A comprehensive flow chart of the chemical separation can be found in Fig. 3 of the supplement. In a 10 mL Savillex® Beaker, 0.5–0.8 g of Bi metal or bismuthinite were dissolved. Metal samples were dissolved in 5 mL 6 M HNO3; bismuthinite samples were dissolved in 6 mL aqua regia. The samples were evaporated to dryness at 125 °C on a hotplate. The residue was converted into the bromide form using 5 mL HBr (4.5 M) and evaporated to dryness at 125 °C. The remaining residue was dissolved in 5 mL oxidative solution 1. As a model for ultra-traces of Tl, a n.c.a. 204Tl stock solution was used as a radioactive tracer. With addition of 0.2 mL 204Tl solution (approximately 3 × 1012 atoms), the mixture was refluxed for at least 24 h at 125 °C and cooled down to room temperature and stored for at least 1 h. For this study 9 M HBr, instead of 0.2 M HBr (as in [2] for the Pb separation) was used as the oxidative agent to avoid the precipitation of BiOBr. BiOBr may occur as an unsolvable residue in aqueous environment and is only solvable in concentrated HBr. Additionally, due to the usage of 9 M HBr instead of 0.2 M HBr, the recovery rates of 204Tl improved significantly. For column chromatography, a 3 mL PP-pipette was used, prepared with a quartz wool plug. 2 mL wet volume of anionic resin (DOWEX® 1X8 100–200 mesh) was used as a stationary phase. The column was cleaned and equilibrated using subsequently 10 mL of reductive solution 2 and 10 mL of oxidative solution 1. After rinsing and equilibration, the sample was taken up in 6 mL oxidative solution 1 and given onto the resin. The beaker was washed out three times using 6 mL oxidative solution 1. When all Tl was washed onto the resin and Bi was eluted, Tl was eluted in a second step with 10 mL reductive solution 2 until no measurable activity remained on the resin. The beaker and the resin were checked for remaining radioactive content using a portable activity counter (S.E.A.® CoMo 170). The obtained fractions were collected into 15 mL centrifuge PP-vials and transferred into 20 mL PP-LSC vials with addition of 10 mL scintillation cocktail. The fractions were analyzed using LSC (Beckman Instuments Inc.® LS 6500 Analyzer). A reference sample of 204Tl was prepared using 0.2 mL 204Tl (aq.) in 10 mL Scintillation cocktail. Additionally, a background reference was prepared using 10 mL scintillation cocktail. The samples were measured in the “Single Rack Mode”. When using the single rack mode, the LSC counts every measurable event, regardless to the radioactive nuclide. The only measurable source of β radiation was 204Tl, which was verified by the background measurement. Details on the LSC measurements are given in Table 3 of the supplement.
Selection of suitable geological mineral samples
Several bismuthinite crystals have been obtained commercially (see supplement Tables 1 and 2), in order to test the feasibility of the Bi–Tl isotope system as a geochronometer. Each bismuthinite sample was screened for its Tl content using LA-ICP-MS (Resonetics® Resolution M50E 193 nm ArF Excimer Laser). However, this technique can only deliver a first estimation since each output value corresponds to an individual laser-spot on the surface of the sample, with cylindric volume of approximately 314 mm3 (50 μm radius and approx. 30–40 μm depth). To account for inhomogenities, each sample was measured 10 times. Following the procedure outlined in Longerich et al. [8], the count rates of 203Tl and 205Tl were normalized to those of an internal standard, which was either 209Bi for Bi2S3 or 33S for samples that had pyrite.
First application of the separation technique to bismuthinite samples and subsequent trace analysis
0.498 g bismuthinite from Karaganda/Kazakhstan and 0.477 g bismuthinite from Tazna/Bolivia were dissolved in 6 mL aqua regia and refluxed for 24 h at 125 °C in a Savillex® beaker. Aqua regia was used for these approaches to ensure a complete dissolution of the bismuthinite and its matrix elements. After evaporation to dryness, the remaining solid was dissolved in 6 mL HBr (4.5 M) and evaporated to dryness. From this point, the separation procedure was followed as described above (see flow chart in Fig. 1 of the supplement). The obtained Tl fractions were further processed for ICP-MS measurements strictly following the procedure as given in [2]. The samples were stored at 6 °C. Immediately before the ICP-MS measurement, the samples were evaporated until only a small drop remained which was taken up in 6 mL HNO3 (0.4 M). The samples were investigated for their Tl content using ICP-MS (Thermo Fisher® X-Series II). Each sample was measured at different dilution factors to prove consistency of the measurements. The calibration was performed using a prepared reference sample of 1 ppm Tl (dilution factor 100). Details on these measurements are given in Table 4 of the supplement. To check the accuracy of the calibration, the certified TMDA-51.4 reference material with a Tl concentration of 20.4 ± 1.7 μg/L was measured. Additionally, blanks of the used acids and H2O samples were investigated for any Tl contamination, yielding evidence that Tl impurities were in all cases below the detection limit of the ICP-MS device. The detection limit for Tl of the Thermo Fisher® X-Series II was determined according to DIN32645: LOD = 0.006 μg/L; LOQ = 0.010 μg/L.
Results and discussion
Development of a separation procedure for the system Bi/Tl and proof of principle using 204Tl as a radioactive tracer
Our results show, that the procedure of Rehkämper could be successfully adapted for the separation of Tl from Bi. The chromatography, averaged over 8 different separations is given in Fig. 1. Additionally.the values are given in the supplementary information (Table 3) in counts per minute (CPM) with the corresponding recovery rates rr ([%]). The values of the 204Tl reference samples (Tlref) for the different approaches are listed there as well.
The uncertainties are calculated from the count rates (2σ). The recovery rates in the experiment ranged from 76 to 96% (average 92%), and the main 204Tl activity was found in fraction 8 with two exceptions where the main activities were found in fractions 7 and 9, respectively. These differences are probably due to differences in the column packing, but the results show that the method is robust if all three fractions 7–9 are collected as Tl fraction.
Selection of suitable geological mineral samples
The suitability of sample minerals depends on their individual Tl content compared to their geological age to maximize the expected radiogenic excess ε205Tl. The radiogenic excess is commonly given as ε within the geological context, with ε = 1 referring to an enhancement of the isotopic ratio of 1/10,000 with respect to the natural composition. The isotopic shift to 205Tl due to α-decay expected of 209Bi would only be recognizable using samples of a high geological age and low impurities of natural Tl. The radiogenic excess of 205Tl can be calculated using the common formula (1):
209Bi decay leads to 205Tl. If the material contains already primordial traces of Tl, which cannot be excluded, the Tl isotopic ratio is shifted from the natural ratio Rnat
to higher values Rdec
Rdec and Rnat refer to the calculated and the natural isotopic ratio of Tl in context to the geological sample age. 203Tl(o) and 205Tl(o) refer to the original isotope composition of Tl. 205Tl(rad) refers to the estimated radiogenic excess of the isotope 205Tl. For the preselection, the geological age of the bismuthinite samples is essential. To maximize the expected radiogenic excess, the samples should be as old as possible and to confirm Tl traces as low as possible. For any primordial trace Tl contents, an expected ε can be calculated depending on the age, as illustrated in Fig. 2. The radiogenic excess depends on both primordial Tl concentration and age. For example a sample with a primordial Tl concentration of 100 ppb will show an excess of ε = 1 after ~ 200 Ma or ε = 5 after ~ 1000 Ma. While ε = 0.5 is measurable with MC-ICP-MS, the natural variation of ε due to fractionation/mineralization process is in the range of ε = −2.12 ± 1.73 up to ε = 11.7 ± 1.3 [2]. Hence for an unbiased determination of the radiogenic excess, ε > 5 is desirable. Even without discussing geological context, deviations with ε > 15 will be highly significant for quantification of Bi α-decay. Taking geological context into account, also samples with ε > 5 are estimated to be sufficient.
The results of the LA-ICP-MS- analysis are shown in Fig. 3. Because pyrite occurred as small intergrowths of FeS2 and Bi2S3, the signal obtained during LA-ICP-MS analysis of these volumes was representative of a mixture of the Tl content of both phases, which made it challenging to use the right internal standard and evaluate the data.
In Fig. 4 the measured Tl concentrations of the samples are plotted against the deposit age to illustrate under which conditions an isotopic analysis of the Tl content for 209Bi α-decay determination is promising.
All samples contain a significant Tl content. Noticeable is the minor scattering of the values of the Tazna- and the Karaganda sample as well as the minor Tl content of the Tazna sample. These are ideal features for an isotopic analysis; therefore, these 2 samples were chosen for digestion and a more precise determination of the bulk Tl content (see below). Unfortunately, the geological sample age of the Tazna sample is not in the significant range to identify an isotopic shift due to the α-decay of 209Bi. It is notable that, with the exception of the Kirchberg sample, all bismuthinite samples consist of grown crystals ≥ 1 cm and contain lower amounts of Tl (Tazna: 0.021 ppm; Sorata: 0.022 ppm; Pechtelsgrün: 0.027 ppm). For the samples from Kirchberg, Karaganda and Tazna, the calculated radiogenic excess of 205Tl is below significance and probably below the measuring scope of MC-ICP-MS (ε205Tl < 1). The radiogenic excess of Sorata, Pechtelsgrün and Manitoba is about 1 < ε205Tl < 5. For these samples, an estimated isotopic excess of 205Tl is in range of attribution to a radiogenic origin and is placed within the scope of a geological evaluation. The Manitoba sample is interesting regarding its geological deposit age. Unfortunately, this sample has insufficient material for an isotopic analysis. Therefore, only the general Tl content was investigated with LA-ICP-MS to determine whether bismuthinite samples from this region are suitable candidates for an isotopic analysis. As it is shown in Fig. 2, for a precise attribution of ε205Tl to a radiogenic origin, geological sample ages of > 8 × 108 years with Tl contents < 20 ppb would be optimal (ε205Tl = 15). To conclude, bismuthinites of a sufficient geological age were found, which are not as homogenous as they need to be for an isotopic analysis. For all samples, the radiogenic excess is probably just minor and thus insufficient for an estimation of the half-life of 209Bi. For Pechtelsgrün and Sorata, the results show some single values with ε > 5, but the samples seem to be inhomogeneous. This corresponds to the enlarged scattering of the Tl concentration values of these samples, since other samples seem to be more homogeneous. The samples with minor scattering values of the Tl content contain of enlarged crystals. Especially the Tazna sample shows low Tl content. The Tazna sample would be an ideal candidate for a half-life estimation of 209Bi if it would be older. The Manitoba sample would be suitable with a low content of Tl and sufficient sample material. We are confident that there are suitable Tl samples, which both contain a requisite low Tl content and show a significant sample age. The samples in our collection are not suitable for an isotope analysis. For the final test of the separation procedure, the Tazna and Karaganda samples were chosen, since the scattering of the Tl concentrations of the Tazna- and Karaganda sample was just minor so these samples were considered homogeneous. Although the Manitoba sample could in principle also be a promising candidate, it was not included because it was estimated that the total amount of bismuthinite in this sample was insufficient for an isotopic measurement. In contrast to the laser ablation procedure, the separation technique delivers bulk-concentrations for Tl. Therefore, the separation procedure shall verify the results of the LA-ICP-MS analysis and will be a final test for the chemistry.
First application of the separation technique to bismuthinite samples and subsequent trace analysis
The LA-ICP-MS technique described above is limited by the fact, that only several local spots in a specific area on the crystal surface can be investigated for pre-testing. But the distribution of the Tl content within the crystal is not necessarily homogenous. A complete digestion of the crystals and subsequent separation and ICP-MS-investigation was performed to clearify the pre-test results, to demonstrate the investigation of the integral Tl content. This would be an important prerequisite regarding the investigation of an isotopic shift to 205Tl.
The isotopic ratio was calculated for the investigated bismuthinite samples (Tazna and Karaganda) as described before (see formula 1). The deviations between pre-testing with LA-ICP-MS and the ICP-MS analysis performed on the digested samples are shown in the following illustrations (Figs. 5 and 6).
While the Tl content of the Tazna sample is slightly higher than estimated on base of the LA-ICP-MS measurements, it is significantly lower in the Karaganda sample. This is a hint that the previously measured Tl might mainly be found at the sample surface. However, in both cases, the Tl content is unfortunately beyond the desired radiogenic range. However, in general, bismuthinites with a significant low natural Tl content are available, e.g. from Vickeroy mine (Zimbabwe), with a geological age of 2.7 × 109 and a Tl content in range of 100 ppt [9]. So although it was not possible in this study to quantify Bi α-decay, we are confident that suitable geological samples can be found.
Conclusions
In this study, an established separation procedure for the system Pb/Tl by Rehkämper et al. was modified and successfully adapted on the system Bi/Tl. Ultra-traces of Tl were successfully simulated using n.c.a. amounts of 204Tl and analyzed using LSC. The procedure was verified by an application to geological samples. Generally, the usage of gaseous components as redox reagents was very successful in avoiding contamination with Tl which was never observed during the study. The method provides a robust separation, reproducibility, high recovery rates and delivers bulk concentrations of Tl. The separation technique was successfully applied for the ultra-trace analysis of Tl in bismuthinites using ICP-MS for analysis. It was yet not possible to obtain specific material, which fulfills simultaneously both needed criteria for an isotopic analysis (old geological age as well as low Tl content). However, samples meeting these criteria separately have been identified. In consequence, although it was yet not possible in this study to quantify Bi α-decay, we are confident that suitable geological samples can be found.
References
de Marcillac P, Coron N, Dambier G, Leblanc J, Moalic JP (2003) Nature 422:876–878
Rehkämper M, Halliday AN (1999) Geochim Cosmochim Acta 63:935–944
Rehkämper M, Frank M, Hein JR, Porcelli D, Halliday A, Ingri J, Liebetrau V (2002) Earth Sci Lett 197:65–81
Nielsen SG, Rehkämper M, Halliday AN (2006) Geochim Cosmochim Acta 70:2643–2657
Beu K (2015) Diploma thesis, University of Cologne
Zendelovska D, Stafilov T (2001) Anal Sci 17:425
Zhou T, Zhang L, Yuan F, Fan Y, Cooke DR (2010) Earth Sci Front 17:306 (tab 1 in English)
Longerich H, Jackson SE, Günther D (1996) J Anal Atom Spectrom 11:899–904
Oberthür T, Weiser TW (2008) Miner Mag 72:953
Acknowledgements
Open Access funding provided by Projekt DEAL. For additional support we want to thank Dr. Peter Sprung (Paul Scherrer Institut, Villigen, Switzerland) and the working group of Prof. Dr. Carsten Münker of the Institute for Geology and Mineralogy/University of Cologne as well as Prof. Dr. Mark Rehkämper of the Imperial College London.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beu, K., Strub, E., Fonseca, R.O.C. et al. Development of a Bi/Tl separation scheme for the proof of 209Bi α-decay in old mineral samples. J Radioanal Nucl Chem 325, 357–363 (2020). https://doi.org/10.1007/s10967-020-07267-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07267-6