Abstract
93Mo is an important long-lived radionuclide in nuclear waste, and is required to be measured during the characterization of decommissioning waste. However, no commercial 93Mo solution is available to be calibrated and used as standard in the analysis of nuclear waste. This work presents a method for separation of 93Mo from Nb metal used in cyclotron as a target holder and irradiated with protons for long time. The separation of 93Mo from Nb matrix was implemented by combination of precipitation and chromatographic separation. The Nb matrix was first removed by precipitating oxides-hydroxides of Nb (e.g. Nb2O5) and then by Fe(OH)3 co-precipitation; Mo in the solution was purified using an alumina (Al2O3) column. A decontamination factor of ca. 105 was achieved for Nb. A pure carrier-free 93Mo solution was successfully prepared, and the 93Mo purity was verified by liquid scintillation spectrometry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
93Mo is produced by neutron activation of stable 92Mo (14.65% of abundance) within the structural materials of nuclear reactors. With progress in nuclear decommissioning activities, determination of 93Mo in nuclear wastes (especially decommissioning wastes) is required in the waste characterization and management. However, 93Mo is still not well characterized and investigated; its half-life [(4.0 ± 0.8) × 103 years [1]] is reported with a relatively high (20%) uncertainty.
93Mo decays by electron capture, and does not emit any γ-line that can be easily measured by γ-spectrometry. It is mostly detected by X-ray spectrometry through its X-rays of 16.521 keV, 16.615 keV, 18.607 keV and 18.623 keV (which are the Kα2, Kα1, Kβ3 and Kβ1 lines of decay product Nb, respectively) [2,3,4,5]. However, 93mNb also emits these X-rays, in the same ratio as 93Mo. Therefore, complete separation of 93Mo from 93mNb before measurement of 93Mo is of paramount importance [5, 6]. The Mo/Nb ratios in the Mo fraction separated from zircaloy hulls from a nuclear reactor were reported to be 100–1000 [6].
By detection of its Auger-electrons, 93Mo can also be measured by liquid scintillation counting (LSC). The major advantages of LSC over X-ray spectrometry for measurement of 93Mo are better availability, higher detection efficiency and lack of self-absorption. Nevertheless, the measurement of 93Mo using LSC is scare; only very few reported LS spectra of 93Mo are available [7, 8].
In addition, a standard solution of 93Mo is not commercially available, even a 93Mo solution is not accessible. Therefore, the preparation of a high purity 93Mo solution is an important issue in the determination and investigation of 93Mo. This work aims to separate 93Mo from a proton irradiated 93Nb (natural Nb) target.
The similar chemical properties of Mo and Nb makes the separation of trace amount of 93Mo from the proton irradiated Nb bulk a major challenge. Only a few methods were reported for separation of 93Mo from niobium matrix. Sadeghi [9] reported a solvent extraction procedure for separation of Mo from NbO2 target solution, and obtained a Mo purity of > 99.9%. Das [10] reported a method for separation of Mo from Nb, Zr and Y using solvent extraction; but this method is mainly dedicated to separate large excess of Mo from trace amounts of Nb, Zr and Y.
Both Mo and Nb readily form anionic species, which behave usually similarly on anion exchange chromatography, and are not retained on cation exchange resin [11,12,13,14]. Their distribution coefficients on anion exchange resin differ significantly only in 40% HF media [15] due to their different behavior in the formation of anion with fluoride. However, it was reported that the recovery of Mo was unsatisfying using a method based on this feature [16]. Shimada reported two methods to separate Mo from — among others — Nb using TEVA resin [5, 17]. The major drawback of these methods is the unsatisfying decontamination of Nb in the Mo fraction.
Butement [18] separated Nb from Mo by co-precipitating Nb on Fe(OH)3. After adding Fe3+ to the solution, he adjusted pH to 10 with NH3·H2O. With this method, more than 90% of Nb was precipitated, and less than 0.5% Mo was observed in the precipitate. As several ions are co-precipitated on Fe(OH)3, similar methods are often used to purify Mo [19] (but no explanation was found in the literature). Bombard [2] applied such a method to separate Mo from Nb in 4 M NH3·H2O medium. Although some (≈ 10%) losses of Mo were observed, the separation was successful, as a decontamination factor (DF) of 1.98 × 104 was reported for Nb after two precipitation-filtration-separation cycles. Puech [20] also used this method and supposedly reported a very similar DF (1.8 × 104) [21].
Alumina (Al2O3) is well known to retain Mo (both as MoO42− and Mo7O246−) in diluted HNO3 (pH = 3.0 ± 0.5) [22, 23], which is widely used in production of 99Mo/99mTc-generators. However, no data about the behavior of Nb on alumina column, or about the behavior of Mo or Nb on alumina column in HF medium have been reported.
For separation of Mo from irradiated Nb, a high DF is needed to get rid of the large amount of Nb matrix (including 93mNb, 94Nb and 94mNb), whereas no reported method meets this requirement. This work aims to develop an effective method for separation of Mo from Nb in order to prepare a pure 93Mo solution from irradiated Nb target.
Experimental
Reagents and materials
Analytical grade reagents were used.
Ca. 35 mg irradiated Nb was taken from a Nb target holder of a GE Healthcare PETtrace 800 cyclotron, which has been used and exposed to proton (ca. 14 MeV) for a long time; so 93Mo was expected to be produced by the reaction of 93Nb(p, n)93Mo.
Single element standard solutions (10 μg/mL) of Mo and Nb were purchased from LabKings, and used as carrier in the chemical separation as well as calibration standards for ICP-AES measurement.
99.8% Nb powder and pieces of 99.95% Mo wire (both purchased from Alfa Aesar) were used in the process of method development.
Alumina column was prepared by loading 2 g of neutral activated alumina (≤ 100 mesh, Brockmann), which was soaked in water, in a standard TrisKem column (7 mm diameter × 6 cm length). The active length of column was 50 mm. The prepared column was rinsed with 20 mL distilled water (to remove the finest particles) and conditioned with 10 mL 0.05 M HF (or another appropriate concentration, corresponding to the loading solution).
Model experiments (using stable Mo and Nb metals)
Typically 25–50 mg Nb and ca. 16 μg Mo were dissolved in the mixture of 1 mL 40% HF and 1 mL 68% HNO3. Solution was evaporated to dryness, the residue was dissolved with 0.5 mL 40% HF, which was evaporated to dryness again. 5 mL 6 M HF was added to the residue and the beaker was warmed slightly, until the residue was completely dissolved.
Distribution of Mo between alumina and HF acid was studied in both batch and column models. In the batch experiments, 1 g alumina was added to 10 mL HF solution of different concentration with the same amounts of Mo (16 μg). Suspensions were shaken for 2 min and kept for overnight (> 12 h). The suspension was centrifuged to separate the supernatant. Mo concentrations in the supernatants were measured.
In the chromatographic experiments, Mo and Nb were prepared in 0.02 M, 0.05 M, 0.1 M and 0.5 M HF solutions, which were loaded onto conditioned alumina columns. After rinsing the columns with 5 mL HF (whose concentrations were the same as those of the sample solutions) and then with 10 mL distilled water, Mo was eluted using 20 mL 1 M NH3·H2O.
Radiochemical separation of Mo from Nb
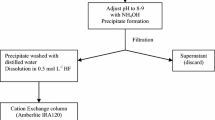
Ca. 15 mg irradiated Nb was transferred to a Teflon beaker, and dissolved in the mixture of 1 mL 40% HF and 1 mL 68% HNO3. 30 μg Mo carrier (3 mL standard solution) was added, and the solution was evaporated to dryness. After adding 0.5 mL 40% HF, it was evaporated to dryness again. 5 mL 6 M HF was added, and the beaker was covered and warmed slightly until the residue was completely dissolved. The solution was transferred into a 50 mL plastic centrifuge tube and diluted with distilled water until 25 mL.
10 mL 25% NH3·H2O was added to form white Nb2O5 precipitate. The sample was shaken for 5 min, and then centrifuged (4 min, 3000 rpm). The supernatant was transferred into another tube and 5 mL solution of FeCl3 (16 mg/mL Fe) was added to form brown Fe(OH)3 precipitate. The supernatant obtained after shaking and centrifugation was transferred into a Teflon beaker, evaporated to dryness 3 times after adding a mixture of ca. 1 mL 36% HCl and ca. 1 mL 68% HNO3, then 3 times after adding ca. 1 mL 40% HF. It was dissolved in 5 mL 0.1 M HF, and 5 mL distilled water was added.
The solution was loaded onto a conditioned alumina column, followed by rinsing with 10 mL 0.05 M HF and then with 10 mL distilled water. Mo was eluted with 10 mL 6.7 M NH3·H2O, the eluate was evaporated gently to dryness, and the residue was dissolved in 5 mL distilled water. A 200 μL aliquot was taken and diluted with 3 mL 3% HNO3 for the measurement of stable Mo and Nb by ICP-AES to calculate chemical recovery of Mo and DF of Nb. 20 mL Ultima Gold LLT cocktail was added to the remaining Mo fraction for the LSC measurement of 93Mo. A schematic diagram of the separation procedure is presented in Fig. 1.
The other half of the irradiated Nb (ca. 15 mg) was processed following the same procedure, but no carrier (stable Mo) was added.
Measurements
ICP-AES (Varian Vista, with AX CCD equipped with ICP Expert II (Agilent Vista PRO Instrument) software) was used for determination of stable element concentration. For measurement of Mo, its 201.512 nm, 202.032 nm, 203.846 nm, 204.598 nm, 268.414 nm, 277.539 nm, 281.615 nm and 284.824 nm lines were used. For measurement of Nb, its 210.942 nm, 229.568 nm, 269.706 nm, 272.198 nm, 294.154 nm, 295.088 nm, 309.417 nm and 313.078 nm lines were used. Standard solutions of Mo and Nb were diluted to the range of 10-1000 ng/mL using 3% HNO3 for calibration of the instrument.
As HF damages the glass pieces of the instrument, samples with high (> 0.5 M) concentration of HF were diluted using 3% HNO3. To solutions containing less (≤ 0.5 M) HF, 0.5 M H3BO3 was added in stoichiometric quantity to complex the excess HF [24]. It has to be taken into account that boric acid increases the number of false signals in the 201.512 nm and 203.846 nm lines for Mo measurement. Components of chemicals used in the separation procedure also interfere with some of the lines, such as Fe with the 201.942 nm line of Nb or Al with the 281.615 nm line of Mo [25].
LSC spectra were acquired using a Wallac Quantulus 1220 ultra low level liquid scintillation spectrometer, and the spectra analysis was implemented using EasyView software.
Results and discussion
Removal of niobium by precipitation of Nb2O5
In the model experiments, almost all initial Mo (≥ 98%) and (9 ± 6)% of initial Nb was found in the supernatant during the Nb2O5 precipitation step. (91 ± 6)% of Nb was precipitated, which is firmly better, than that reported by Espartero [26] with only 50–60% being precipitated, and 46–92% reported by Osváth [27] using a similar procedure. These results indicate that precipitation of Nb2O5 can be used as a pre-concentration step for removal of most of Nb, but the DF (≈ 10) is insufficient.
Removal of niobium by precipitation of Fe(OH)3
The preliminary experiments showed that precipitation of Fe(OH)3 in water (adding 25% NH3·H2O dropwise until pH ≈ 8) caused both Nb and Mo being co-precipitated, and only 11–15% of Mo was found in the supernatant. On the contrary, when a much bigger volume of 25% NH3·H2O was added and Fe(OH)3 was precipitated in 2 M, 4 M or 6 M NH3·H2O solution (pH ≫ 8), most of Mo (92 ± 9)%, but only (0.31 ± 0.16)% of Nb occurred in the supernatant. This indicates that the concentration of NH3·H2O plays an important role in the separation of Mo from Nb in this step. Similar results were reported using 6 M NH4C2H3O2 [19] or 4 M NH3·H2O [2]. It is not clear why high concentration of NH3·H2O can prohibit Mo to be precipitated or adsorbed on the precipitate of hydroxides. It might be attributed to the formation of soluble MoO42−, as Mo can form MoO42− in alkaline solution, but not in neutral, acidic or slightly alkaline solution [28]. According to another possible explanation, the excess NH3·H2O forms some soluble complexes with Mo. Anyway no correlation between concentration of NH3·H2O and DF of Nb was found, indicating that this step is not sensitive to the concentration of NH3·H2O.
It was observed that Mo in the supernatant from the hydroxide precipitation could not be efficiently adsorbed on alumina column when the supernatant was directly loaded onto the column. A good retention of Mo was achieved by evaporating the supernatant to dryness, evaporating it repeatedly after adding a mixture of 36% HCl and 68% HNO3, then evaporating it repeatedly after adding 40% HF, and finally re-dissolving the residue in diluted HF solution. This probably is a consequence of the chemical form of Mo: a possible ammine complex has to be decomposed; but further investigation is needed to clearly explain the phenomenon.
Loading Mo onto alumina column
The batch experiments (Table 1) show that Mo was quantitatively adsorbed on the alumina (> 99%) when HF concentration was lower than 0.05 M. When the HF concentration was increased to 0.1–0.5 M, some of Mo occurred in the supernatant.
The column experiments (Table 2) show that both Mo and Nb were well retained on alumina, when the loading solution was prepared in 0.02–0.1 M HF (Nb was also well retained in 0.5 M HF).
It was also observed that most of Mo (> 95%) could be adsorbed on the alumina column when the solution was prepared in diluted HNO3 (< 1 M). However, some small fractions of Mo (up to 7%) occurred sometimes in the effluent. Importantly, various fractions of Nb (up to 51%) also occurred in the effluent, indicating that Nb could not be well retained on the alumina column.
No leakage of Mo or Nb was observed when column was rinsed with water. However, both Mo and Nb (retained from HF) have leaked when column was rinsed with 0.1–1 M HNO3. Therefore 0.05 M HF was finally selected as loading and rinsing solution.
Eluting Mo from alumina column
For elution of Mo, 1 M NH3·H2O is typically used [29, 30]; but highest recovery (≥ 80%) was achieved using concentrated (25% or 13.4 M) NH3·H2O [31]. Our results (Table 3) show that the more concentrated the NH3·H2O eluent, the more efficient the elution of Mo. However, total recoveries of Mo are within 86–99% (being typically over 92%). Concentrations of Nb in the eluate were below detection limit in all the Mo fractions, indicating that the DF of Nb is higher than 104.
Performance of the complete separation procedure
Two simulated solutions with high Nb and low Mo concentration were treated using the developed procedure. The results (Table 4) show that more than 75% of Mo can be separated, and DFs of Nb are higher than 3x105. The separation of 93Mo from the irradiated Nb target holder sample shows a relatively low recovery of only 48%. The slightly lower DF of ≥ 8.9 × 104 for Nb is estimated, this is attributed to the detection limit of Nb in the eluate of Mo.
Measurement using LSC
LSC spectrum of the separated 93Mo is presented in Fig. 2. Peak of 93Mo was found in channels 25–325. This is in good agreement with the reported LSC spectra of 93Mo [7, 8]. Spectral quench parameter (SQP) of spectrum was 828. According to Roos [32], this is corresponding to (52 ± 5)% detection efficiency, which gives an activity of (26.4 ± 2.6) Bq.
Conclusions
A fast and reliable procedure was developed to separate Mo from irradiated Nb. The method is based on the combination of precipitation of Nb2O5, co-precipitation with Fe(OH)3 as well as alumina column separation. Purity of separated Mo was checked by ICP-AES; Mo recovery was 48%, DF of Nb was ≥ 8.9 × 104. LSC spectrum of 93Mo was acquired. Our results indicate that the developed separation procedure can be used for preparation of standard solution of 93Mo.
References
Chu SYF, Ekström LP, Firestone RB (1999) The Lund/LBNL nuclear data search (version 2.0, February 1999). http://nucleardata.nuclear.lu.se/toi/. Accessed 16 Jan 2019
Bombard A (2005) Dosage de radionucléides à vie longue, 93Zr, 93Mo et 94Nb, dans des échantillons issus de l’industrie nucléaire. Thesis, Nantes University, France
Remenec B, Dulanská S, Mátel L (2013) Determination of difficult to measure radionuclides in primary circuit facilities of NPP V1 Jaslovske Bohunice. J Radioanal Nucl Chem 298:1879–1884. https://doi.org/10.1007/s10967-013-2679-4
Remenec B, Dulanská S, Mátel L (2014) Determination of alpha, beta, X-ray and gamma emitting radionuclides in reactor components and fuel assemblies from NPP V1 Jaslovske Bohunice. J Radioanal Nucl Chem 299:1799–1804. https://doi.org/10.1007/s10967-013-2904-1
Shimada A, Kameo Y (2016) Development of an extraction chromatography method for the analysis of 93Zr, 94Nb, and 93Mo in radioactive contaminated water generated at the Fukushima Daiichi Nuclear Power Station. J Radioanal Nucl Chem 310:1317–1323. https://doi.org/10.1007/s10967-016-5008-x
Bertaux M, Bienvenu P, Provitina O, Point C, Bilcot J-B, Brochard E, Pontremoli S, Arnal N (2008) Analytical improvements for long-lived radionuclides determination in Zircaloy Hulls. In: ATALANTE 2008, Montpellier (France), May 19–22. http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/40/015/40015638.pdf. Accessed 6 Feb 2017
Ermakov AI, Malinovsky SV, Kashirin IA, Tikhomirov VA, Sobolev AI (2005) Rapid analysis of radionuclide composition (screening) of liquid samples via deconvolution of their LS spectra. In: Chalupnik S, Schönhofer F, Noakes J (eds) LSC 2005, Advances in liquid scintillation spectrometry, Radiocarbon, University of Arizona, pp 89–98
Roos P (2019) Isolation and quantification of a 93Mo isotope solution from proton irradiated niobium. Talanta 204:769–775. https://doi.org/10.1016/j.talanta.2019.06.042
Sadeghi M, Enferadi M, Nadi H, Tenreiro C (2010) A novel method for the cyclotron production no-carrier-added 93mMo for nuclear medicine. J Radioanal Nucl Chem 286(1):141–144. https://doi.org/10.1007/s10967-010-0622-5
Das MK, Das SS, Madhusmita Nayer MA, Chattopadhyay S, Barua L, Siddhartha Datta S (2017) Separation of Mo from Nb, Zr and Y: applicability in the purification of the recovered enriched 100Mo used in the direct production of 99mTc in cyclotrons. J Radioanal Nucl Chem 311(1):643–647. https://doi.org/10.1007/s10967-016-5000-5
Fritz JS, Garralda BB, Karraker SK (1961) Cation exchange separation of metal ions by elution with hydrofluoric acid. Anal Chem 33(7):882–886
Massart DL (1971) Cation-exchange techniques in radiochemistry (NAS-NS 3113). National Academy of Sciences, National Research Council. https://www.osti.gov/servlets/purl/4699019. Accessed 22 Feb 2018
Caletka R, Krivan V (1983) Cation-exchange of 43 elements from hydrofluoric acid solution. Talanta 30(7):543–545. https://doi.org/10.1016/0039-9140(83)80128-3
Dulanská S, Bilohuščin J, Remenec B, Mátel L, Silliková V (2016) Sequential determination of 93Zr, 94Nb, 99Tc and 126Sn in radioactive waste using anion exchange resin and TEVA Resin. J Radioanal Nucl Chem 309:685–689. https://doi.org/10.1007/s10967-015-4613-4
Lederer M (1992) The periodical table for chromatographers. Wiley, Chichester
Caletka R, Krivan V (1983) A group separation based on anion- and cation-exchange from hydrofluoric acid medium. Application to multielement neutron-activation analysis of niobium. Talanta 30(7):465–470. https://doi.org/10.1016/0039-9140(83)80111-8
Shimada A, Ozawa M, Yabuki K, Kimiyama K, Sato K, Kameo Y (2014) Development of a separation method for molybdenum from zirconium, niobium, and major elements of rubble samples. J Chromatogr A 1371:163–167. https://doi.org/10.1016/j.chroma.2014.10.053
Butement FDS, Qaim SM (1964) New radioisotopes of niobium and molybdenum—II. 88Mo and 89Mo. J Inorg Nucl Chem 26:1491–1501. https://doi.org/10.1016/0022-1902(64)80036-1
Scadden EM, Ballou NE (1960) Radiochemistry of molybdenum. http://library.lanl.gov/cgi-bin/getfile?rc000069.pdf. Accessed 20 May 2013
Puech P, Bienvenu P (1998) Détermination des radionucléides zirconium 93 et molybdène 93 dans des effluents de retraitement des combustibles irradiés. Direction du cycle du combustible, Rapport scientifique 1998. http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/33/034/33034331.pdf. Accessed 21 Oct 2008
Puech P (1998) Détermination des radionucléides zirconium 93 et molybdène 93 dans des effluents de retraitement des combustibles irradiés. Thesis, Univ. Paris XI, 211, France (Impossible to find it)
Molinski VJ (1982) A review of 99mTc generator technology. Int J Appl Radiat Isot 33:811–819. https://doi.org/10.1016/0020-708X(82)90122-3
Arino H, Kramer HH (1975) Fission product 99mTc generator. Int J Appl Radiat Isot 26:301–303. https://doi.org/10.1016/0020-708X(75)90165-9
Wu S, Zhao YH, Feng X, Wittmeier A (1996) Application of inductively coupled plasma mass spectrometry for total metal determination in silicon-containing solid samples using the microwave-assisted nitric acid–hydrofluoric acid–hydrogen peroxide–boric acid digestion system. J Anal At Spectrom 11:287–296. https://doi.org/10.1039/JA9961100287
Osváth Sz (2017) ICP-OES measurement of some transition metals in HF acid media. In: NKS-B ICP user seminar—development and exploration of inductively coupled plasma spectrometry, Roskilde (Denmark), September 25–27. http://www.nks.org/download/ICP_2017_pdfs/szabolcs_nks-icp-sem-2017_v06.pdf. Accessed 30 Jun 2019
Espartero AG, Suárez JA, Rodrígez M (1988) Determination of 93mNb and 94Nb in medium and low level radioactive wastes. Appl Radiat Isot 49(9–11):1277–1282. https://doi.org/10.1016/S0969-8043(97)10059-8
Osváth Sz, Vajda N, Molnár Zs (2008) Determination of long-lived Nb isotopes in nuclear power plant wastes. Appl Radiat Isot 66:24–27. https://doi.org/10.1016/j.apradiso.2007.07.007
Tkac P, Paulenova A (2008) Speciation of molybdenum(VI) In aqueous and organic phases of selected extraction systems. Sep Sci Technol 43(9–10):2641–2657. https://doi.org/10.1080/01496390802122261
Bernhard G (1994) Separation of radionuclides from a fission product solution by ion exchange on alumina. J Radioanal Nucl Chem 177(2):321–325. https://doi.org/10.1007/BF02061128
Lehto J, Hou X (2011) Chemistry and analysis of radionuclides: laboratory techniques and methodology. Wiley-VCH Verlag & Co KGaA, Weinheim
Wilkinson MV, Mondino AV, Manzini A (2002) Study of alumina use as a separation step in Mo-99 production. http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/35/015/35015845.pdf. Accessed 15 Sept 2017
Roos P (2018) Calibration of a 93Mo standard. In: NKS-B Rad Workshop, radioanalytical chemistry for nuclear decommissioning and waste management, Roskilde, Denmark, October 8–12
Acknowledgements
Open access funding provided by National Center for Public Health. The authors acknowledge prof. Mikael Jensen at Hevesy Laboratory, DTU Nutech for providing proton irradiated niobium sample, and Dr. Per Roos and other colleagues at Radioecology Section, Hevesy Laboratory, DTU Nutech for their kind help during the experiments. The first author is particularly grateful for the assistance given by the staff of Hevesy Laboratory, DTU Nutech.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Osváth, S., Qiao, J. & Hou, X. Preparation of 93Mo solution using proton irradiated Nb. J Radioanal Nucl Chem 322, 1833–1839 (2019). https://doi.org/10.1007/s10967-019-06758-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06758-5