Abstract
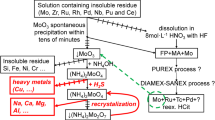
Uranium-free molybdenum-based CerMet fuels can be applied for transmutation of minor actinides in GEN IV reactors or ADS. In a reprocessing concept designed in the first paper in this mini-series (Mareš and John in J Radioanal Nucl Chem 320(1):227–233, 2019. https://doi.org/10.1007/s10967-019-06456-2) the residual radionuclidic impurities need to be separated from slightly alkaline (pH 9.1) highly concentrated ammonium molybdate solutions. Screening tests of technetium extraction onto three extraction chromatographic materials were performed in present study. Solid extractants based on Aliquat® 336 turned out to be the most promising for Tc extraction from molybdenum solutions both from the point of view of the extraction kinetics and the practical extraction capacity. The overall results of this mini-series allowed to conclude that if the molybdenum recycling concept proposed is adopted, separation of the residual radionuclidic impurities should not represent a significant problem.




Similar content being viewed by others
References
Mareš KV, John J (2019) Recycling of isotopically modified molybdenum from irradiated CerMet nuclear fuel: part 1—concept design and assessment. J Radioanal Nucl Chem 320(1):227–233. https://doi.org/10.1007/s10967-019-06456-2
Dehaudt P, Mocellin A, Eminet G, Caillot L, Delette G, Bauer M, Viallard I (1997) Composite fuel behaviour under and after irradiation. In: Studies on fuel with low fission gas release. IAEA-TECDOC-970, pp 23–33, ISSN 1011-4289
Calabrese R, Vettraino F, Artioli C, Sobolev V, Thetford R (2010) Heterogeneous fuels for minor actinides transmutation: fuel performance codes predictions in the EFIT case study. Ann Nucl Energy 37(6):867–874
Haas D, Fernandez A, Staicu D, Somers J, Maschek W, Liu P, Chen XN (2008) CerMet fuel behaviour and properties in ADS reactors. Energ Convers Manag 49(7):1928–1933
Fernández A, Konings RJM, Somers J (2003) Design and fabrication of specific ceramic-metallic fuels and targets. J Nucl Mater 319:44–50
Fernández A, Haas D, Konings RJM, Somers J (2004) Transmutation of actinides. J Am Ceram Soc 85:694–696
De Visser-Týnová E, Ménard G, Ebert EL, Modolo G, Cheng M, Steppert M, Walther C, Geist A, Mareš KV, John J (2015) Dissolution behaviour of inert matrix fuels. In: Proc. TopFuel 2015, Zürich, CH, September 13–17, 10 pp
Nash K, Lumetta G (2011) Advanced separation techniques for nuclear fuel reprocessing and radioactive waste treatment, Woodhead Publishing, USA, 512 pp. ISBN 978-1-84569-501-9
Petrochenkov VA, Gorichev IG, Batrakov VV, Izotov AD, Kutepov M (2004) Catalytic effec of ammonia on the kinetics and mechanism of MoO3 dissolution in alkaline solutions. Theor Found Chem Eng 38(4):386–393
Mareš KV, Šebesta F, John J (2019) Recycling of isotopically modified molybdenum from irradiated CerMet nuclear fuel: part 2—caesium separation from concentrated molybdate solution. J Radioanal Nucl Chem 320(2):377–384. https://doi.org/10.1007/s10967-019-06480-2
Mareš KV, Šebesta F, John J (2019) Recycling of isotopically modified molybdenum from irradiated CerMet nuclear fuel: part 3—strontium separation from concentrated molybdate solution. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06553-2
Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Maxwell SL III, Nelson M (1995) Separation and preconcentration of actinides by extraction chromatography using a supported liquid anion exchanger: application to the characterization of high-level nuclear waste solutions. Anal Chim Acta 310:63–78
Dulanská S, Bilohuščin J, Remenec B, Mátel L, Silliková V (2015) Sequential determination of 93Zr, 94Nb, 99Tc and 126Sn in radioactive waste using anion exchange and TEVA® Resin. J Radioanal Nucl Chem 309(2):685–689
Long KM, Goff GS, Ware SD, Jarvinen GD, Runde WH (2012) Anion exchange resins for the selective separation of technetium from uranium in carbonate Solutions. Ind Eng Chem Res 51(31):10445–10450
Jarvinen GD, Long KM, Goff GS, Runde WH, Maulsolf EJ, Czerwinski KR, Poineau F, McAlister DR, Horwitz EP (2013) Separation of pertechnetate from uranium in simulated UREX processing solution using anion exchange extraction chromatography. Solvent Extr Ion Exch 31(4):416–429
Collins JL, Egan BZ, Anderson KK, Chase CW, Bell JT (1995) Batch test equilibration studies examining the removal of Cs, Sr, and Tc from supernatants from ORNL underground storage tanks by selected ion exchangers. In: Proc. International Conference of Waste Managemant, 17 pp
Lee CH, Suh MYS, Jee KY, Kim WH (2007) Sequential separation of 99Tc, 94Nb, 55Fe, 90Sr and 59/63Ni from radioactive waste. J Radioanal Nucl Chem 272(1):187–194
Masud RS, Wu Y, Mimura H, Niibori Y, Onishi T, Koyama S (2012) Selective separation of Re(VII) and Tc(VII) by xerogel microcapsules enclosing TOA extractant. Procedia Chem 7:258–267
Stepinski D, Bennett M, Naik SR, Ling L, Wang NHL, Vandegrift GF (2016) Development of ABEC column for separation of Tc-99 from NorthStar dissolved target solution. Argonne National Laboratory, 22 pp, Report No. ANL/NE-16/43
Paučová V, Remenec B, Dulanská S, Mátel Ľ, Prekstová M (2012) Determination of 99Tc in soil samples using molecular recognition technology product AnaLig® Tc-02 gel. J Radioanal Nucl Chem 293:675–677
Remenec B, Dulanská S, Paučová Mátel Ľ (2011) Determination of 99Tc in evaporator concentrates using solid phase extraction techniques. J Radioanal Nucl Chem 290:403–407
Uchida S, Tagami K (1997) Separation and concentration of technetium using a Tc-selective extraction chromatographic resin. J Radioanal Nucl Chem 221(1–2):35–39
Lučaníková M, Kučera J, Šebesta F (2008) New extraction chromatographic material for rhenium separation. J Radioanal Nucl Chem 277(2):479–485
Gumiela M, Dudek J, Bilewicz A (2016) New Precipitation Method for Isolation of 99mTc from Irradiated 100Mo Target. J Radioanal Nucl Chem 310:1061–1067
Yu S, Chen J (1985) Mechanism of synergistic extraction of rhenium(VII) by primary amies and neutral phosphorus esters. Hydrometallurgy 14:115–126
Zhan-fang C, Hong Z, Zhao-hui Q (2009) Solvent extraction of rhenium from molybdenum in alkaline solution. Hydrometallurgy 97:153–157
Mann NR, Wood DJ, Todd TA, Šebesta F (2009) U.S. Patent 7,629,292 B2
IBC Advanced Technologies: AnaLig gel data sheet technetium column series Tc-02. IBC Advanced Technologies, Inc
TEVA® resin (1995) Eichrom analytical products description. Eichrom Industries, Inc.
Cheresnowsky MJ (1988) Process for producing ammonium molybdate from molybdenum trioxide. US Patent 4,735,791
Babu BV, Gupta S (2004) Modeling and simulation for dynamics of packed bed adsorption. In: Proceedings of international symposium & 57th annual session of IIChE in association with AIChE (CHEMCON-2004), Mumbai, 27–30 December 2004
Acknowledgements
This study was supported by the EU 7th Framework Programme project ASGARD (EC-GA No. 295825) focusing on research into advanced/novel fuels fabrication and their reprocessing issues for Generation IV reactors, by the Grant Agency of the Czech Technical University in Prague (Grants Nos. SGS12/199/OHK4/3T/14 and SGS15/216/OHK4/3T/14), and by the Centre for Advanced Applied Science, Project Number CZ.02.1.01/0.0/0.0/16_019/0000778, supported by the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mareš, K.V., Daňo, M., Šebesta, F. et al. Recycling of isotopically modified molybdenum from irradiated CerMet nuclear fuel: part 4—technetium separation from concentrated molybdate solution. J Radioanal Nucl Chem 321, 775–781 (2019). https://doi.org/10.1007/s10967-019-06622-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06622-6