Abstract
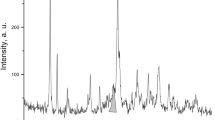
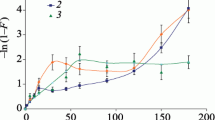
The parameters of kinetics of Sr2+ sorption by natural clinoptilolite were investigated. The overall rate constants were determined by application of pseudo-first and pseudo-second order kinetic models. The actual sorption mechanism was studied by application of both intraparticle and surface film diffusion models. The application of Rietveld structure refinement shows the preferable sites of strontium ion exchange in the clinoptilolite structure and their diffusion through cationic sites with time, as well as indicates the positions occupied by Sr2+ ions at the start of the exchange process and the positions where Sr2+ accumulates in subsequent ion exchange stages.






Similar content being viewed by others
References
Alby D, Charnay C, Heran M, Prelot B, Zajac J (2018) Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: synthesis and shaping, sorption capacity, mechanisms, and selectivity—a review. J Hazard Mater 344:511–530
Delkash M, Bakhshayesh BE, Kazemian H (2015) Using zeolitic adsorbents to cleanup special wastewater streams: a review. Microporous Mesoporous Mater 214:224–241
Misaelides P (2011) Application of natural zeolites in environmental remediation: a short review. Microporous Mesoporous Mater 144:15–18
Lihareva N, Petrov O, Tzvetanova Y, Kadiyski M, Nikashina V (2015) Evaluation of the possible use of a Bulgarian clinoptilolite for removing strontium from water media. Clay Miner 50:55–64
Lihareva N, Petrov O, Tzvetanova Y (2017) Modelling of Cs+ uptake by natural clinoptilolite from water media. Bulg Chem Commun 49:577–582
Ho YS, Ng JCY, McKay G (2000) Kinetic of pollutant sorption by biosorbents: review. Sep Purif Methods 29:189–232
Ho YS, McKay G (1999) Pseudo-second-order model for sorption processes. Process Biochem 34:451–465
Weber WJ Jr, Morris JC (1963) Kinetic of adsorption on carbon from solution. J Sanitary Eng Div 89:31–38
Boyd GE, Adamson AW, Mayers LS (1947) The exchange adsorption of ions from aqueous solutions by zeolites. II. Kinetics. J Am Chem Soc 69:28–36
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2:65–71
Topas V 4.2: general profile and structure analysis software for powder diffraction. Bruker AXS, Karlsruhe
Ho YS, McKay G (1999) The sorption of lead(II) on peat. Water Res 33:578–584
Wu F-Ch, Tseng R-L, Huang R-S (2001) Kinetic modelling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res 35:613–618
Ofomaja AE (2010) Intraparticle diffusion process for lead(II) biosorption onto mansonia wood sawdust. Bioresour Technol 101:5868–5876
Gupta SS, Bhattacharyya K (2006) Adsorption of Ni(II) on clays. J Colloid Interface Sci 295:21–32
Chen H, Wang A (2007) Kinetic and isotherm studies of lead adsorption onto palygorskite clay. J Colloid Interface Sci 307:309–316
Aroua MK, Leong SP, Teo LY, Yin CY, Daud WM (2008) Real-time determination of kinetics of adsorption of lead(II) onto palm shell-based activated carbon using ionselective electrode. Bioresour Technol 99:5786–5792
Cheung WH, Szeto YS, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98:2897–2904
Kragović M, Selulić Ž, Stojanović M, Petrović M, Dondur V, Damjanović L, Jović A (2014) Kinetic of Pb(II) ions removal from aqueous solution using the Fe(III)-modified zeolite. In: Zeolite 2014, book of abstracts of the 9th international conference of the occurrence, properties and utilization of natural zeolites, Belgrade, 8–13 June 2014, pp 109–110
Ofomaja AE (2008) Kinetic study and sorption mechanism of methylene blue and methyl violet onto mansonia (Mansonia altissima) wood sawdust. Chem Eng J 143:85–95
Waranusantigul P, Pokethitiyook P, Kruatrachue M, Upatham ES (2003) Kinetic of basic dye (methylene blue) biosorption by giant duckweed (Soirodela polyrrhiza). Environ Pollut 125:385–392
Kumar KV, Ramamurthi V, Sivanesan S (2005) Modeling the mechanism involved during the sorption of methylene blue onto fly ash. J Colloid Interface Sci 284:14–21
Allen SJ, McKay G, Khader KY (1989) Intraparticle diffusion of a basic dye during adsorption onto sphagnum peat. Environ Pollut 56:39–50
Wang S, Li H, Hu L (2006) Application of zeolite MCM-22 for basic dye removal from wastewater. J Colloid Interface Sci 295:71–78
Reichenberg D (1953) Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. J Am Chem Soc 75:589–597
El-Kamash AM (2008) Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J Hazard Mater 151:432–445
Koyama K, Takeuchi Y (1977) Clinoptilolite: the distribution of potassium atoms and its role in thermal stability. Z Kristallogr 145:216–239
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lihareva, N., Dimowa, L., Petrov, O. et al. Study of the kinetics and mechanism of Sr2+ sorption by clinoptilolite. J Radioanal Nucl Chem 321, 31–38 (2019). https://doi.org/10.1007/s10967-019-06574-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06574-x