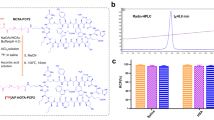
Abstract
Accumulation of triphenylphosphonium (TPP) is normally observed in the mitochondria from the extracellular spaces due to the high difference in plasma membrane potential of the mitochondria. In the cancer cells, the mitochondrial membrane potential gap is higher as compared to that of normal cells resulting in elevated uptake of TPP. Silica nanoparticles (SNPs) being widely developed and used for biomedical applications, in this study, we tried to modify the surface of SNPs with varying amounts of TPP to verify their possible application as a positron emission tomography (PET) agent. The studies confirmed that the high level of TPP loading on the surface of SNPs possess higher positive charge (+ 31.5 mV). Owing to this behavior of plasma membrane potential of cancerous cells the uptake of positively charged SNPs was much higher in tumor cells than that of normal cells which was confirmed by PET imaging.







Similar content being viewed by others
References
Cho K, Wang X, Nie S, Shin DM (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14:1310–1316
Faraji AH, Wipf P (2009) Nanoparticles in cellular drug delivery. Bioorgan Med Chem 17:2950–2962
Zhang C, An T, Wang D, Wan G, Zhang M, Wang H, Zhang S, Li R, Yang X, Wang Y (2016) Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly (β-amino ester)/poly (lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J Control Release 226:193–204
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nanotechnology 2:751–760
Kim J, Cao L, Shvartsman D, Silva EA, Mooney DJ (2011) Targeted delivery of nanoparticles to ischemic muscle for imaging and therapeutic angiogenesis. Nano Lett 11:694
Kim K-R, Kim HY, Lee Y-D, Ha JS, Kang JH, Jeong H, Bang D, Ko YT, Kim S, Lee H (2016) Self-assembled mirror DNA nanostructures for tumor-specific delivery of anticancer drugs. J Control Release 243:121–131
Kingsley JD, Dou H, Morehead J, Rabinow B, Gendelman HE, Destache CJ (2006) Nanotechnology: a focus on nanoparticles as a drug delivery system. J Neuroimmune Pharm 1:340–350
Tang L, Cheng J (2013) Nonporous silica nanoparticles for nanomedicine application. Nano Today 8:290–312
Lee SB, Kim HL, Jeong HJ, Lim ST, Sohn MH, Kim DW (2013) Mesoporous silica nanoparticle pretargeting for PET imaging based on a rapid bioorthogonal reaction in a living body. Angew Chem Int Edit 52:10549–10552
Lee H, Sung D, Kim J, Kim B-T, Wang T, An SSA, Seo S-W, Yi DK (2015) Silica nanoparticle-based dual imaging colloidal hybrids: cancer cell imaging and biodistribution. Int J Nanomed 10:215
Keinänen O, Mäkilä EM, Lindgren R, Virtanen H, Liljenbäck H, Oikonen V, Sarparanta M, Molthoff C, Windhorst AD, Roivainen A (2017) Pretargeted PET imaging of trans-cyclooctene-modified porous silicon nanoparticles. ACS Omega 2:62
Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S (2011) Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest 121:2768
Bradbury MS, Phillips E, Montero PH, Cheal SM, Stambuk H, Durack JC, Sofocleous CT, Meester RJ, Wiesner U, Patel S (2013) Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr Biol 5:74–86
Smith RA, Hartley RC, Cocheme HM, Murphy MP (2012) Mitochondrial pharmacology. Trends Pharmacol Sci 33:341–352
Marrache S, Pathak RK, Dhar S (2014) Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc Natl Acad Sci 111:10444–10449
Kwon HJ, Cha M-Y, Kim D, Kim DK, Soh M, Shin K, Hyeon T, Mook-Jung I (2016) Mitochondria-targeting ceria nanoparticles as antioxidants for alzheimer’s disease. ACS Nano 10:2860–2870
Zhang XY, Zhang P-Y (2016) Mitochondria targeting nano agents in cancer therapeutics. Oncol Lett 12:4887–4890
Smith RA, Porteous CM, Gane AM, Murphy MP (2003) Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci 100:5407–5412
Frantz MC, Wipf P (2010) Mitochondria as a target in treatment. Environ Mol Mutagen 51:462–475
Gruber J, Fong S, Chen C-B, Yoong S, Pastorin G, Schaffer S, Cheah I, Halliwell B (2013) Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv 31:563–592
Davis S, Weiss M, Wong J, Lampidis TJ, Chen LB (1985) Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J Bio Chem 260:13844–13850
Dairkee SH, Hackett AJ (1991) Differential retention of rhodamine 123 by breast carcinoma and normal human mammary tissue. Breast Cancer Res Treat 18:57–61
Modica-Napolitano JS, Aprille JR (2001) Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev 49:63–70
Sarparanta M, Mäkilä E, Heikkilä T, Salonen J, Kukk E, Lehto V-P, Santos HA, Hirvonen J, Airaksinen AJ (2011) 18F-labeled modified porous silicon particles for investigation of drug delivery carrier distribution in vivo with positron emission tomography. Mol Pharm 8:1799–1806
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interf Sci 26:62–69
Rahman IA, Padavettan V (2012) Synthesis of silica nanoparticles by sol–gel: size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—a review. J Nanomater. https://doi.org/10.1155/2012/132424
Sato-Berrú R, Saniger JM, Flores-Flores J, Sanchez-Espíndola M (2013) Simple method for the controlled growth of SiO2 spheres. J Mat Sci Eng A 3:237–242
Bhatt NB, Pandya DN, Jide XuJ, Tatum D, Magda D, Wadas TJ (2017) Evaluation of macrocyclic hydroxyisophthalamide ligands as chelators for zirconium-89. PLoS ONE 12:1–13
Acknowledgements
Our study was supported by the Nuclear R&D Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (2015M2A2A4A02043265 and 2016M2C2A1937989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, G.G., Lee, J.Y., Choi, P.S. et al. Synthesis and evaluation of triphenylphosphonium conjugated 18F-labeled silica nanoparticles for PET imaging. J Radioanal Nucl Chem 316, 1099–1106 (2018). https://doi.org/10.1007/s10967-018-5763-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5763-y