Abstract
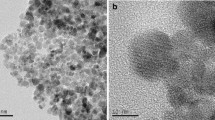
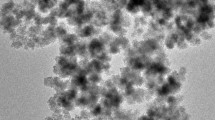
The in situ formed magnetite nanoparticles was encapsulated by maleated chitosan to synthesize a novel magnetic chitosan nano-sorbent (MCN-MA) for the effective sorption of uranium. The sorption kinetics could be described by the pseudo-second-order model, whereas the sorption isotherms could be fitted to the Langmuir model (q m = 187.9 mg/g). The MCN-MA showed higher U(VI) sorption capacities (compared to MCN) due to high affinity of carboxylate groups introduced from grafting maleic anhydride. Thermodynamic parameters indicate that U(VI) sorption is endothermic and feasible. The nano-size and magnetic property of the MCN-MA allow its efficient U(VI) sorption and facile magnetic separation from wastewaters.









Similar content being viewed by others
References
Wang X, Fan Q, Yu S, Chen Z, Ai Y, Sun Y, Hobiny A, Alsaedi A, Wang X (2016) High sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations. Chem Eng J 287:448–455
Elwakeel KZ, Atia AA (2014) Uptake of U(VI) from aqueous media by magnetic Schiff’s base chitosan composite. J Clean Prod 70:292–302
Gu B, Liang L, Dickey MJ, Yin X, Dai S (1998) Reductive precipitation of uranium(VI) by zero-valent iron. Environ Sci Technol 32:3366–3373
Khayambashi A, Wang X, Wei Y (2016) Solid phase extraction of uranium(VI) from phosphoric acid medium using macroporous silica-based D2EHPA-TOPO impregnated polymeric adsorbent. Hydrometallurgy 164:90–96
Hoyer M, Zabelt D, Steudtner R, Brendler V, Haseneder R, Repke JU (2014) Influence of speciation during membrane treatment of uranium contaminated water. Sep Purif Technol 132:413–421
Sole KC, Cole PM, Feather AM, Kotze MH (2011) Solvent extraction and ion exchange applications in Africa’s resurging uranium industry: a review. Solvent Extr Ion Exch 29:868–899
Aziz A, Jan S, Waqar F, Mohammad B, Hakim M, Yawar W (2010) Selective ion exchange separation of uranium from concomitant impurities in uranium materials and subsequent determination of the impurities by ICP-OES. J Radioanal Nucl Chem 284:117–121
Galhoum AA, Mahfouz MG, Atia AA, Abdelrehem ST, Gomaa NA, Vincent T et al (2015) Amino acid functionalized chitosan magnetic nano-based particles for uranyl sorption. Ind Eng Chem Res 54:12374–12385
Yin X, Bai J, Tian W, Li S, Wang J, Wu X (2017) Uranium sorption from saline lake brine by amidoximated silica. J Radioanal Nucl Chem 313:1–9
Hritcu D, Humelnicu D, Dodi G, Popa MI (2012) Magnetic chitosan composite particles: evaluation of thorium and uranyl ion adsorption from aqueous solutions. Carbohydr Polym 87:1185–1191
Wang JS, Peng RT, Yang JH, Liu YC, Hu XJ (2011) Preparation of ethylenediamine-modified magnetic chitosan complex for adsorption of uranyl ions. Carbohydr Polym 84:1169–1175
Pan D, Fan Q, Fan F, Tang Y, Zhang Y, Wu W (2017) Removal of uranium contaminant from aqueous solution by chitosan@ attapulgite composite. Sep Purif Technol 177:86–93
Galhoum AA, Mahfouz MG, Gomaa NM, Vincent T, Guibal E (2017) Chemical modifications of chitosan nano-based magnetic particles for enhanced uranyl sorption. Hydrometallurgy 168:127–134
Xu J, Chen M, Zhang C, Yi Z (2013) Adsorption of uranium(VI) from aqueous solution by diethylenetriamine-functionalized magnetic chitosan. J Radioanal Nucl Chem 298:1375–1383
Elwakeel KZ, Atia AA, Guibal E (2014) Fast removal of uranium from aqueous solutions using tetraethylenepentamine modified magnetic chitosan resin. Bioresour Technol 160:107–114
Sutirman ZA, Sanagi MM, Karim KJ, Wan AW (2016) Preparation of methacrylamide- functionalized crosslinked chitosan by free radical polymerization for the removal of lead ions. Carbohydr Polym 151:1091–1099
Kyzas GZ, Siafaka PI, Lambropoulou DA, Lazaridis NK, Bikiaris DN (2014) Poly(itaconic acid)-grafted chitosan adsorbents with different cross-linking for Pb(II) and Cd(II) uptake. Langmuir 30:120–131
Reddy DH, Lee SM (2013) Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv Colloid Interface Sci 202:68–93
Li N, Bai R (2006) Highly enhanced adsorption of lead ions on chitosan granules functionalized with poly(acrylic acid). Ind Eng Chem Res 45:7897–7904
Ogawa N, Honmyo K, Harada K, Sugii A (2010) Preparation of spherical polymer beads of maleic anhydride–styrene–divinylbenzene and metal sorption of its derivatives. J Appl Polym Sci 29:2851–2856
Shaker MA, Yakout AA (2016) Optimization, isotherm, kinetic and thermodynamic studies of Pb(II) ions adsorption onto N-maleated chitosan-immobilized TiO2 nanoparticles from aqueous media. Spectrochim Acta A 154:145–156
Zhou Y, Hu X, Jin Q, Wang X, Ma T (2013) Adsorption of Cd(II) from aqueous solutions by cellulose modified with maleic anhydride and thiourea. Adsorpt Sci Technol 31:583–598
Sarkar K, Kundu PP (2012) Preparation of low molecular weight N-maleated chitosan graft-PAMAM copolymer for enhanced DNA complexation. Int J Biol Macromol 51:859–867
Sureshkumar MK, Das D, Mallia MB, Gupta PC (2010) Adsorption of uranium from aqueous solution using chitosan tripolyphosphate (CTPP) beads. J Hazard Mater 184:65–72
Yusupkhanova M, Shaposhnikova ST, Aikhodzhaev BI (1980) Determination of the carboxyl groups in a copolymer of styrene with maleic anhydride. Fibre Chem 11:417–419
Simsek S, Yilmaz E, Boztug A (2013) Amine-modified maleic anhydride containing terpolymers for the adsorption of uranyl ion in aqueous solutions. J Radioanal Nucl Chem 298:923–930
Monier M, Ayad DM, Wei Y, Sarhan AA (2010) Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin. J Hazard Mater 177:962–970
Rahmati A, Ghaemi A, Samadfam M (2012) Kinetic and thermodynamic studies of uranium(VI) adsorption using Amberlite IRA-910 resin. Ann Nucl Energy 39:42–48
Wang GH, Liu JS, Wang XG, Xie ZY, Deng NS (2009) Adsorption of uranium (VI) from aqueous solution onto crosslinked chitosan. J Hazard Mater 168:1053–1058
Liu Y, Cao X, Hua R, Wang Y, Liu Y, Pang C, Wang Y (2010) Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy 104:150–155
Hosoba M, Oshita K, Katarina RK, Takayanagi T, Oshima M, Motomizu S (2009) Synthesis of novel chitosan resin possessing histidine moiety and its application to the determination of trace silver by ICP-AES coupled with triplet automated-pretreatment system. Anal Chim Acta 639:51–56
Das D, Sureshkumar MK, Koley S, Mithal N, Pillai C (2010) Sorption of uranium on magnetite nanoparticles. J Radioanal Nucl Chem 285:447–454
Zhang X, Jiao C, Wang J, Liu Q, Li R, Yang P, Zhang M (2012) Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: kinetic and thermodynamic investigation. Chem Eng J 198:412–419
Stopa L, Yamaura M (2010) Uranium removal by chitosan impregnated with magnetite nanoparticles: adsorption and desorption. Int J Nucl Energy Sci Technol 5:283–289
Donia AM, Atia AA, Moussa EM, El-Sherif AM, El-Magied MOA (2009) Removal of uranium(VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95:183–189
Wang H, Ma L, Cao K, Geng J, Liu J, Song Q, Yang X, Li S (2012) Selective solid phase extraction of uranium by salicylideneimine-functionalized hydrothermal carbon. J Hazard Mater 229:321–330
Acknowledgements
This work was financially supported by the National Natural Science Foundation (21366001; 21667001; 21601033), the International Scientific and Technological Cooperation Projects (2015DFR61020), the Key Research and Development Program and the Natural Science Fund Program of Jiangxi Province (20161BBF60059; S2017ZRMSB0473; GJJ161583).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shehzad, H., Zhou, L., Li, Z. et al. Effective adsorption of U(VI) from aqueous solution using magnetic chitosan nanoparticles grafted with maleic anhydride: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 315, 195–206 (2018). https://doi.org/10.1007/s10967-017-5647-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5647-6