Abstract
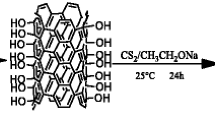
Multiwalled carbon nanotubes (MWCNTs) were modified by strong oxidizing agents and were functionalized with toluene 2,4-diisocyanate, and they were used for selective separation of Tl-201 from Pb-201 (radioactive lead). The pristine and functionalized MWCNTs were characterized by Fourier transform infrared spectroscopy and scanning electron microscopy. The optimal conditions of experiment, such as pH, amount of adsorbent, and contact time were investigated. The adsorption capacity was evaluated using both Langmuir and Freundlich adsorption isotherm models. The results showed that functionalized MWCNTs have a greater potential for adsorption of lead from aqueous solution, nuclear sample, and separation of Tl-201 from Pb-201.














Similar content being viewed by others
References
Jia YX, Lee CS, Zettl A (1994) Stabilization of the Tl2Ba2Ca2Cu3O10 superconductor by Hg doping. Phys. C 234:24–28
Minami C, Takei K, Funahashi T, Kubota H (1990) Recovery of high purity Thallium at sumitomo works. Rare Met Int 90:259–262
Marczenko Z (1986) Separation and spectrophotometric determination of elements. E. Horwood, Chichester, pp 564–571
Luke CL (1959) Photometric determination of antimony and thallium in lead. Anal Chem 31:1680–1682
Sato T, Suzuki K, Sato K (1989) Solvent extraction of trivalent Gallium, Indium, and Thallium from hydrochloric acid solutions by an alpha-hydroxyoxime. In: Proc Int Conf. on Sep Sci Technol, vol 2. Ottawa, pp 539–547
Sato T, Sato K (1989) Solvent extraction of trivalent Aluminum, Gallium, Indium, and Thallium from hydrochloric acid solutions by an acid organophosphorus compounds. In: Proc Int Conf. on Sep Sci Technol, vol 2.Ottawa, pp 567–577
Sato T, Sato K (1992) Liquid-liquid extraction of Indium(III) from aqueous acid solutions by acid oroganophosphorus compounds. Hydrometallurgy 30:367–383
Sato T, Yasumura H, Mizuno Y, Nishimura T (1996) Solvent extraction of trivalent Gallium, Indium, and Thallium from hydrochloric acid solutions by TOPO and TBP. Solvent Extr 1:559–564
Sato T, Sato K, Noguchi Y, Ishikawa I (1997) Liquid–liquid extraction of trivalent Gallium, Indium and Thallium from hydrochloric acid solutions by tributyl phosphate and trioctylamine. J Min Mat 113:185–192
Sodd VJ, Scholz KL, Blue JW (1982) Separation of Thallium-201 from Lead-201 using N-benzylaniline. J Radioanal Chem 68:277–280
Albert L, Masson H (1994) Thallium extraction process. Google Patents 1994
Nozaki T (1956) Indirect colorimetric determination of Thallium. J Chem Soc Jpn Pure Chem Sect 77:493–498
Sttrelow FWE, Victor AH (1972) Quantitative separation of Al, Ga, In, and Tl by cation exchange chromatography in hydrochloric acid-acetone. Talanta 19:1019–1023
Matthews AD, Riley JP (1969) The determination of Thallium in silicate rocks, marine sediments and sea water. Anal Chem Acta 48:25–34
Means JL, Crerar DA, Borcsik MP, Duguid JO (1978) Adsorption of Co and selected actinides by Mn and Fe oxides in soils and sediments. Geochim Cosmochim Acta 42:1763–1773
Lin TS, Nriagu JO (1998) Speciation of Thallium in natural waters. In: Nriagu JO (ed) Thallium in the environment, advances in environmental sci. and tech, vol 29, Wiley, New York pp 31–44
Rauws A, Canton J (1976) Adsorption of Thallium ions by Prussian Blue. Bull Environ Contam Toxicol 15:335–336
Srivastava S, Bhattacharjee G (1980) Studies in the use of inorganic gels in the removal of heavy metals. Water Res 14:113–115
Eyde D (1993) Using zeolites in the recovery of heavy metals from mining effluents. In: Hager JP (ed) EPD Congress’ 93, Proceedings EPD-TMS Annual Meeting, Denver, CO, 1993, The minerals, metals, and materials society, Warrendale, 1993, pp 383–392
Zaitseva N, Deptula C, Khan K, Knotek O, Mikeć P, Khalkin V (1988) Radiochemical separation of radio Thallium from proton-irradiated Lead. J Radioanal Nucl Chem 121:307–321
Yamini Y, Ashtari P, Khanchi AR, Ghanadi-Maragheh M, Shamsipur M (1999) Preconcentration of trace amounts of uranium in water samples on octadecyl silica membrane disks modified by bis(2-ethylhexyl) hydrogen phosphate and its determination by alpha-spectrometry without electrodeposition. J Radioanal Nucl Chem 242:783–786
Shamsipur M, Yamini Y, Ashtari P, Khanchi AR, Ghanadi-Maragheh M (2000) A rapid method for the extraction and separation of uranium from thorium and other accompanying elements using octadecyl silica membrane disks modified by Tri-n-octyl phosphine oxide. Sep Sci Technol 35:1011–1019
Ashtari P, Wang K, Yang X, Ahmadi SJ (2009) Preconcentration and separation of ultra-trace Beryllium using quinalizarine modified magnetic microparticles. Anal Chim Acta 646:123–127
Zheng F, Baldwin DL, Fifield LS, Anheier NC, Aardahl CL, Grate JW (2006) Single-walled carbon nanotube paper as a sorbent for organic vapor preconcentration. Anal Chem 78:2442–2446
Zhou QX, Wang WD, Xiao JP (2006) Preconcentration and determination of nicosulfuron, thifensulfuron-methyl and metsulfuron-methyl in water samples using carbon nanotubes packed cartridge in combination with high performance liquid chromatography. Anal Chim Acta 559:200–206
Zhou QX, Jp Xiao, Wang WD, Liu GG, Shi QZ, Wane JH (2006) Determination of atrazine and simazine in environ- mental water samples using to high performance liquid chromatography with diode array detector. Talanta 68:1309–1315
Liang P, Ding Q, Song F (2005) Application of multiwalled carbon nanotubes as solid phase extraction sorbent for preconcentration of trace copper in water samples. J Sep Sci 28:2339–2343
Liang P, Liu Y, Guo L, Zeng J, Lu HB (2004) Multiwalled carbon nanotubes as solid-phase extraction adsorbent for the preconcentration of trace metal ions and their determination by inductively coupled plasma atomic emission spectrometry. J Anal Atom Spectr 19:1489–1492
Li Y, Wang S, Luan Z, Ding J, Xu C, Wu D (2003) Adsorption of cadmium(II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 41:1057–1062
Tavallali H, Fakhraee V (2011) Preconcentration and determination of trace amounts of Cd2+ using multiwalled carbon nanotubes by solid phase extraction-flame atomic absorption spectrometry. Int J ChemTech Res 3:1628–1634
Pu Y, Yang X, Zheng H, Wang D, Su Y, He J (2013) Adsorption and desorption of Thallium(I) on multiwalled carbon nanotubes.Chem. Eng J 219:403–410
Tavallali H (2013) Preconcentration and determination of trace amounts of Ag(I) and Pb(II) using multiwalled carbon nanotubes by solid phase extraction-flame atomic absorption spectrometry. Int J Chem Tech Res 3(3):1628–16345
Biaduń E, Sadowska M, Ospina-Alvarez N, Krasnodębska-Ostręga B (2016) Direct speciation analysis of Thallium based on solid phase extraction and specific retention of a Tl(III) complex on alumina coated with sodium dodecyl sulfate. Microchim Acta 183(1):177–183
Rosengrant L, Craig RM (1990) Final best demonstrated available technology (BDAT) background document for P and U Thallium wastes. US EPA Office of Solid waste, Washington, p 19
Asadollahi N, Yavari R, Ghanadzadeh H (2015) Preparation, characterization and analytical application of stannic molybdophosphate immobilized on multiwalled carbon nanotubes as a new adsorbent for the removal of strontium from wastewater. J Radioanal Nucl Chem 303:2445–2455
Zhao C, Ji L, Liu H, Hu G, Zhang S, Yang M, Yang Z (2004) Functionalized carbon nanotubes containing isocyanate groups. J Solid State Chem 177:4394–4398
Moosa A, Ridha AM, Najem Abdullha I (2015) Chromium ions removal from wastewater using carbon nanotubes. Int J Innov Res Sci Eng Technol 4(2). doi:10.15680/IJIRSET.2015.0402057
Srivastava S (2013) Sorption of divalent metal ions from aqueous solution by oxidized carbon nanotubes and nanocages: a review. Adv Mater Lett 4(1):2–8
Kim YS, Cho JH, Ansari SG, Kim HI, Dar MA, Seo HK, Kim GS, Lee DS, Khang G, Shin HS (2006) Immobilization of avidin on the functionalized carbon nanotubes. Synth Met 156:938–943
Shimizu K, Phanopoulos C, Loenders R, Abel ML, Watts JF (2010) The characterization of the interfacial interaction between polymeric methylene diphenyl diisocyanate and aluminum: a ToF-SIMS and XPS study. Surf Interface Anal. doi:10.1002/sia.3586
Weng CH (2004) Modeling Pb(II) adsorption onto sandy loam soil. J Collid Interface Sci 272:262–270
Sitko R, Turek E, Zawisza B, Malicka E, Talik E, Heimann J, Gagor A, Feist B, Wrzalik R (2013) Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans 42:5682–5689
Atieh MA, Bakather OY, Tawabini BS, Bukhari AA, Khaled M, Alharthi M, Fettouhi M, Abuilaiwi FA (2010) Removal of Chromium(III) from water by using modified and nonmodified carbon nanotubes. J Nanomater. doi:10.1155/2010/232378
Ruparelia JP, Duttagupta SP, Chatterjee AK, Mukherji S (2008) Potential of carbon nanomaterials for removal of heavy metals from water. Desalination 232:145–156
Tehrani MS, Abroomand Azar P, Ehsani Namin P, Moradi Dehaghi Sh (2013) Removal of Lead ions from wastewater using functionalized multiwalled carbon nanotubes with Tris(2-Aminoethyl)Amine. Appl Environ Prot. doi:10.4236/jep.2013.46062
Tehrani MS, Abroomand Azar P, Ehsani Namin P, Moradi Dehaghi Sh (2014) Removal of Lead ions from aqueous solution using multi-walled carbon nanotubes: the effect of functionalization. J Appl Environ Biol Sci 4:316–326
Bulut E, Ozacar M, Sengil IA (2008) Equilibrium and kinetic data and process design for adsorption of congo red onto bentonite. J Hazard Mater 154:613–622
Mohammadi S, Afzali D, Pourtalebi D (2010) Flame atomic absorption spectrometric determination of trace amounts of Lead, cadmium and nickel in different matrixes after solid phase extraction on modified multiwalled carbon nanotubes. Cent Eur J Chem 8(3):662–668
Lian N, Chang X, Zheng H, Wang S, Cui Y, Zhai Y (2005) Application of dithizone modified TiO2 nanoparticles in the preconcentration of trace chromium and Lead from sample solution and determination by inductively coupled plasma atomic emission spectrometry. Microchim Acta 151:81–86
Ozer A (2007) Removal of Pb(II) ions from aqueous solutions by sulphuric acid-treated wheat bran. J Hazard Mater 141:753–758
Acknowledgments
The authors thank the Ehsan Maadi and Sedigheh Moradkhani for carrying out Polarography and HPGe studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ouni, L., Mirzaei, M., Ashtari, P. et al. Isocyanate functionalized multiwalled carbon nanotubes for separation of lead from cyclotron production of thallium-201. J Radioanal Nucl Chem 310, 633–643 (2016). https://doi.org/10.1007/s10967-016-4928-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4928-9