Abstract
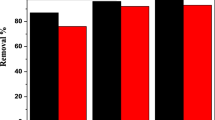
A uranyl sulfate leach liquor obtained by uranium leaching of a technological sample of salcrete deposits of Gabal Qatrani ore was subjected to uranium extraction using the liquid–liquid technique. Uranium was effectively extracted from sulfate leach liquor by [(10 %) tri-n-octylamine (TOA)] dissolved in xylene as a diluent. The extraction efficiency was markedly enhanced as the concentration of TOA increases from 1 to 10 %. The relevant factors controlling the extraction process of uranium using tri-n-octylamine were studied. These factors include the effect of diluents used, TOA concentration, contact time, settling time and phase ratio (O/A) v/v. The optimum extraction conditions were chosen. Stripping of uranium from the loaded TOA has been carried out using 5 % Na2CO3 as an effective stripping agent. More than 97 % of uranium was extracted by 10 % TOA, at contact time 10 min, settling time 5 min, phase ratio (VO/VA) 1/1 and at room temperature. The feasibility of using the TOA for preconcentration-separation of uranium was assessed by stripping studies. The loaded uranium onto TOA has been stripped by 100 % when using 5 % Na2CO3 as an efficient eluting agent at 15 min contact time, 5 min settling time and phase ratio (O/A) 2/1.












Similar content being viewed by others
References
Kumar JR, Kim JS, Lee JY, Yoon HS (2011) A brief review on solvent extraction of uranium from acidic solutions. Sep Purif Rev 40(2):77–125
IAEA meeting, 1979. In: Proceedings of an advisory group meeting organized by the International Atomic Energy Agency, 5–8 June. Production of yellow cake and uranium fluorides, Paris.
Alexander MSJ, Cattrall RW, Kolev SD (2010) Extraction of uranium (VI) from sulfate solutions using a polymer inclusion membrane containing di-(2-ethylhexyl) phosphoric acid. J Membr Sci 364:354–361
Ring RJ (2000) Current practice for the milling of uranium ores, in: Uranium 2000. In: Proceedings of the international symposium on the process metallurgy of uranium, Saskatoon, SK, Canada, pp 851–875.
Harvinsderpal S, Gupta CK (2008) Solvent extraction in production and processing of uranium and thorium
Seidel DC (1979) Extracting of uranium from its ores. IAEA Bull 23(2):24–28
Mackenzie JMW (1997) Uranium solvent extraction using tertiary amines, presented at Uranium Ore Yellow Cake Seminar February Melbourne, Australia, 3–23.
Marczenko Z (1976) Spectrophotometric determination of elements. Ellis Horwood Ltd., Chichester
Robbins LA (1981) Liquid–liquid extraction. In: Schwitzer A (ed) Hand book of separation techniques for chemical engineers. McGraw-Hill, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daher, A.M., Wanees, S.A., Kellah, H.M.A. et al. Removal of uranium from sulfate leach liquor of salcrete deposits using tri-n-octyl amine. J Radioanal Nucl Chem 299, 493–499 (2014). https://doi.org/10.1007/s10967-013-2762-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2762-x