Abstract
A radiochemical procedure is developed for the determination of 237Np in soil with multi-collector inductively-coupled plasma mass spectrometry (MC-ICP-MS) and gamma-spectrometry. 239Np (milked from 243Am) was used as an isotopic tracer for chemical yield determination. The neptunium in the soil is separated by thenoyl-trifluoracetone extraction from 1 M HNO3 solution after reducing Np to Np(IV) with ferrous sulfamate, and then purified with Dowex 1 × 2 anion exchange resin. 239Np in the resulting solution is measured with gamma-spectrometry for chemical yield determination while the 237Np is measured with MC-ICP-MS. Measurement results for soil samples are presented together with those for two reference samples. By comparing the determined value with the reference value of the 237Np activity concentration, the feasibility of the procedure was validated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neptunium-237 (T 1/2 = 2.14 × 106 years) is released to the environment as a result of nuclear weapons tests, reactor accidents and nuclear fuel reprocessing. As a multivalent element, Np may be mobile in certain speciation and migrates into biosphere with underground water. When ingested by human being, Np accumulates in the liver and bones. Therefore, 237Np is regarded as a highly radiologically toxic pollutant due to its alpha particle emission and long half-life. In order to assess its environmental risk and determine its origin, the quantification of 237Np in soil is necessary. Due to the low concentration of 237Np in the environment, preconcentration is usually required before it can be measured with alpha-spectrometry or ICP-MS [1]. 236Pu or 242Pu is usually used as a yield tracer for 237Np because there lack appropriate isotopic tracers for 237Np yield determination [2–4]. Isotopes of Pu are not good tracers for Np because the two elements often fractionate during chemical processing. 235Np is a potential tracer for 237Np [5, 6]. However, 235Np free from contamination of 237Np is not commercially available. 236Np was utilized as the tracer by some researchers to assay 237Np in environmental samples where measurement was carried out with mass spectrometry [7–9]. However, 236Np is not easy to produce and still not available in pure form to most researchers [4]. 239Np has been used as a yield tracer for chemical recovery determination of 237Np in the environmental samples with alpha-spectrometry [6, 10–13]. However, the analysis of 237Np with alpha-spectrometry usually costs too much time due to its low concentration. Such analytical approaches also require additional chemical operations, such as electrolytic deposition. MC-ICP-MS is particularly effective for measurement of long-lived actinide isotopes with lower specific activity, including 237Np. The purpose of this paper is to develop a method for rapid determination of 237Np using 239Np as a yield tracer, in which MC-ICP-MS and gamma-spectrometer are employed to measure 237Np and 239Np, respectively.
Experimental
Preparation of 239Np tracer
239Np in radioactive equilibrium with its 243Am parent was separated with HDEHP extraction chromatography resin (100–200 mesh, Beijing Research Institute of Chemical Engineering and Metallurgy, China) as shown in Fig. 1. Briefly, (1) Add 243Am spike solution of ~105 Bq to a beaker and dilute to 0.1 M HNO3 with deionized water. (2) The solution is passed through a HDEHP extraction chromatography resin column (0.75 cm i.d. × 10 cm long) which is pre-equilibrated with 15 ml 0.1 M HNO3 at a flow rate of 1 ml/min and washed with 10 ml 0.1 M HNO3 to improve the recovery of 239Np. The eluate is collected into a clean vessel. (3) The eluted 239Np in the vessel was measured by gamma-ray spectroscopy to check the yield of this isotope. (4) 243Am was later recovered by washing the column with 20 ml of 1 M HNO3.
Procedure for determination of 237Np in soil
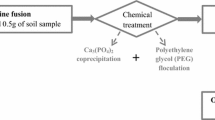
The new procedure for determination of 237Np in environmental soil samples is based on thenoyl-trifluoroacetone (TTA) extraction combined with anion exchange chromatography as illustrated in Fig. 2 and described as following.
-
(1)
The soil sample was pulverized and dried to constant weight in an oven at 110 °C, and then homogenized with a spatula.
-
(2)
Add 1 g of homogenized soil to a clean beaker and ignite at 550 °C in a furnace over night.
-
(3)
Transfer the ignited soil sample to a Teflon beaker. Add 239Np tracer (about 1000 Bq), 20 ml 15 M HNO3, 40 ml 22 M HF and 8 ml 12 M HClO4 to the sample and digest the mixture on a hot plate for 2 h until the soil sample is dissolved and the solution is clear. Evaporate the solution to dryness at 150 °C on a hot plate and then transfer the residue to a 100 ml glass beaker. Ignite the residue for 30 min at 550 °C in a furnace.
-
(4)
Dissolve the residue with 1 M HNO3 solution. Add 1 ml 0.4 M ferrous sulfamate to the solution. Stir it with a glass rod and let it stand for 15 min to reduce Np to IV state completely.
-
(5)
The Np(IV) is now extracted from the aqueous HNO3 solution by contacting it with 5 ml of a 0.5 M solution of TTA in xylene. Use a mechanical shaker for this operation, with a contact time of 15 min. Repeat this operation once with a fresh TTA-xylene solution and combine the two xylene solutions. Strip the combined xylene solution with 10 ml of 1 M HNO3 in the mechanical shaker with a contact time of 10 min. Discard the aqueous phase.
-
(6)
Back-extract Np(IV) from the organic phase twice, each with 5 ml 8 M HNO3 for 10 min. Combined the aqueous phases and wash with 5 ml xylene for 10 min. Discard the organic phase.
-
(7)
Add 1 ml 0.4 M ferrous sulfamate and 0.5 ml hydrazine hydrate to the aqueous solution. Stir it with a glass rod and let it stand for 10 min to reduce Np to IV state completely.
-
(8)
The solution is passed through an anion exchange column (0.5 cm i.d. × 10 cm long) containing Dowex 1 × 2 resin (100–200 mesh, Sigma-aldrich, USA). The resin in the column must be pretreated by passing 10 ml 8 M HNO3 through it with a flow rate of 0.2 ml/min. Wash the column with 8 ml 8 M HNO3 and 5 ml 5 M HNO3 successively to further remove U and other elements in the soil matrix.
-
(9)
Strip Np with 10 ml 0.5 M HNO3 and evaporate the eluate solution to dryness at 150 °C on a hot plate.
-
(10)
The sample is dissolved with 3 ml 0.1 M HNO3 and transferred into a vial for gamma-spectrometric measurement of chemical yield.
-
(11)
The quantity of 237Np was measured with MC-ICP-MS using the standard addition method. After the 3-ml solution of 0.1 M HNO3 is nondestructively analyzed for 239Np by gamma-ray spectrometry, it is divided into six equal aliquots. Five of these aliquots are individually added to five standard solutions of 237Np. The sixth aliquot is added to an equal volume of a solution containing no 237Np. All six solutions are immediately analyzed for 237Np by MC-ICP-MS.
Results and discussion
Purity check of 239Np tracer
The 239Np intended as a tracer for 237Np was obtained from 243Am as described above. In order to assure the suitability of the 239Np as a tracer, its purity was checked with a HPGe gamma-spectrometer. Figure 3 shows the gamma-ray spectrum of 243Am in equilibrium with 239Np before separation. The peaks of both 239Np (277.6 keV) and 243Am (74.7 keV) appear clearly in the spectrum. However, when 239Np was separated from 243Am parent and checked with a HPGe gamma-spectrometer, only the peak of 239Np can be seen in the spectrum and no lines for 243Am are distinguishable in the spectrum (see Fig. 4).
239Np is a short-lived β-emitter (T 1/2 = 2.355 days) while 243Am is a long-lived α-emitter (T 1/2 = 7370 years). The activity equilibrium of this mother/daughter pair is reached after 23.6 days, about 10 half lives of 239Np [14, 15]. Therefore, 239Np can be prepared from the same 243Am solution again and again after the activity equilibrium of this mother/daughter pair is reached.
Validation of the analytical procedure
In order to validate the applicability of the analytical procedure to soil samples with complicated matrix, two reference soil samples R1 (1 g) and R2 (1 g), with known 237Np activity concentration were analyzed for 237Np concentration according to the procedure described above and the results are compared with the reference values in Table 1. The 237Np activity concentration of R1 was 0.040 Bq/g, with a difference of −3.6 % compared to the reference value. The 237Np activity concentration of R2 was 0.050 Bq/g, with a difference of 2.0 % compared to the reference value. It can be seen that the determined results are in good agreement with the reference data for both reference soils, suggesting that the new analytical method applies to soil sample very well.
Application of the proposed procedure in practical environment samples assay
To verify the feasibility of the proposed analytical procedure for practical environment samples, the 237Np activity concentrations of the two real soil samples (namely, S1 and S2) were determined following the proposed procedure. The S1 and S2 surface soil samples (sandy soil) were collected from the northwest of China, near a nuclear facility site. The soils were ground and sieved to get a powder with particle diameters ranging from 74 to 149 μm. The uniformity was checked with gamma spectrometric measurement.
After the separation procedure, the resulting solutions containing purified 237Np and spiked 239Np were transfered to clean tubes and measured with the gamma-spectrometer in the same geometry as the 239Np tracer solution had been measured. By this way, the detection efficiency of the detector is not required to be calibrated because only relative counts in the same equipment are used in calculations. The 277.6 keV photopeak of 239Np was chosen because of its relatively high intensity and its location in a comparatively flat baseline region of the spectrum. The yield of 239Np (denoted Y) is calculated by means of Eq. (1).
where C s is the counts of 239Np in the resulting solution, cps; C 0 is the counts of 239Np in added tracer spike, cps; λ is the the decay constant of 239Np, s−1; t the time gap between the two measurements of 239Np, s.
The yields of 239Np are listed in Table 2 together with the determined 237Np concentration. It can be seen that although the 237Np activity concentration of the two soil samples is very low, the reproducibility of the results is <6 %, which is rather good for environmental analysis. However, the chemical yield is a bit lower due to the complicated matrix of the soil.
Due to the low concentration of 237Np in the soil samples, the measurement with alpha-spectrometry [13] may cost too much time (about 3–4 days) in order to have precise results. Besides, the matrix components, including interfering elements and radionuclides, have to be removed effectively with many difficulties for α measurement. With this new analytical method, it takes 2 days less for measurement with MC-ICP-MS [13]. Methods that use 242Pu [3, 4] as a tracer for 237Np presume that the chemistries of Pu and Np are so similar that Pu and Np follow one another in soil and in the chemical treatment of soil samples. No such assumption is made in the new method because 239Np and 237Np have identical chemistries.
Conclusion
A new rapid separation method was developed for the determination of 237Np in soil samples with MC-ICP-MS and gamma-spectrometry. There are two advantages of the present procedure. One is that it adopts gamma-emitting 239Np instead of 236Pu or 242Pu as the tracer, and the other is that the yield of Np can be determined without relative efficiency calibration of gamma-spectrometer for 239Np sources. The feasibility of the procedure was validated by analyzing the two reference soil samples with known 237Np activity concentration.
References
Baglain N, Bouvier-Capely C, Cossonnet C (2002) Radiochim Acta 90:267
Sill DS, Bohrer SE (2000) Radioact Radiochem 11:7
Chen Q, Dahlgaard H, Nielsen SP (2002) J Radioanal Nucl Chem 253:451
Qiao J, Hou X, Roos P (2010) J Anal At Spectrom 25:1769
Salminen S, Paatero J, Roos P (2009) J Radioanal Nucl Chem 281:405
Harvey BR, Sutton GA (1987) Nucl Instr Meth Phys Res A254:172
Kelley JM, Bond LA, Beasley TM (1999) Sci Total Environ 237(238):483
Cooper LW, Kelley JM, Bond LA (2000) Mar Chem 69:253
Kenna TC (2002) J Anal At Spectrom 17:1471
Harvey BR, Lovett MB (1984) Nucl Instr Meth Phys Res 223:224
Popplewell DS et al (1987) J Radioanal Nucl Chem 115:191
Diodati JM, Sartori FM (2007) J Radioanal Nucl Chem 272:11
Rosa JL, Outola I, Crawford E, Radioanal J (2008) Nucl Chem 277:11
Wenzel U, Bisplinghoff B (2002) J Radioanal Nucl Chem 254:527
Reich M, Rajec P (2005) J Radioanal Nucl Chem 266:71
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Yi, X., Shi, Y., Xu, J. et al. Rapid determination of 237Np in soil samples by multi-collector inductively-coupled plasma mass spectrometry and gamma spectrometry. J Radioanal Nucl Chem 298, 1757–1761 (2013). https://doi.org/10.1007/s10967-013-2677-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2677-6