Abstract
The paper summarizes the results of the 240Pu/239Pu atomic ratio studies in atmospheric fallout samples collected in 1986 over Gdynia (Poland) as well as three Baltic fish species collected in 1997 using the accelerator mass spectrometry. A new generation of AMS has been developed during last years and this method is an efficient and good technique to measure long-lived radioisotopes in the environment and provides the most accurate determination of the atomic ratios between 240Pu and 239Pu. The nuclide compositions of plutonium in filter samples correspond to their means of production. AMS measurements of atmospheric fallout collected in April showed sufficient increase of the 240Pu/239Pu atomic ratio from 0.28 from March to 0.47. Also such high increase of 240Pu/239Pu atomic ratio, close to reactor core 240Pu/239Pu atomic ratio, was observed in September and equaled 0.47.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The measurement of isotopic ratios such as 240Pu/239Pu can provide information on the source of contamination. Most of the environmental studies demand low detection limits for the alpha-emitter 239Pu (T 1/2 = 24 110 a, E = 5,155.5 keV (73.3 %), 5,142.8 keV (15 %), 5,104.7 keV (10 %)), the fissile and the major isotope. In addition to 239Pu, 240Pu (T 1/2 = 6,564 a; E = 5,168.2 keV (73 %), 5,123.7 keV (26 %)), the second major isotope produced via neutron capture of 239Pu, deserves special attention, as the 240Pu/239Pu ratio offers unambiguous information about the plutonium source [1, 2]. However, the measurement of anthropogenic actinides in the environment is difficult, as they are present in the very low concentrations and often have long half-lives, which makes measurements by means of radioactive decay counting difficult or even impossible [2–5]. Alpha spectrometry is commonly used for determination of actinides in environmental samples. This technique requires the radio-tracers and long-lasting as well as laborious work. However, it gives excellent detection limits. Alpha spectrometers typically require 200 keV peak to peak separation for baseline resolution between successive alpha emission of different isotopes [6]. Even with the lowest 18 keV resolution of the typical PIPS detectors used during alpha spectrometry measurements, it is difficult to determine the abundance of 239Pu and 240Pu isotopes in samples because the five primary alpha emissions for the two isotopes lie within a range of 65 keV, with the lowest difference at 13 keV.
Accelerator mass spectrometry (AMS) is a modern analytical technique which allows the long-living radioactive radionuclides to be measured in environmental samples [4, 7–10]. New generation AMS enables the heaviest elements (actinides) to be detected and the isotopic ratios between them to be estimated. Mass-spectrometric methods (e.g. AMS, TIMS, ICP-MS) do give information on the 240Pu/239Pu ratio, and potentially have higher sensitivity than α-particle counting, with values as low as ~1 fg having been reported, but are sensitive to molecular interferences, i.e. 238UH, 208Pb, 31P, etc. could interfere with measurement of 239Pu [11, 12]. AMS is characterized by low measurement background and high detection efficiency; separation of isotopes is possible, but (atomic or molecular) isobars can not be resolved [3, 13, 14].
In the case of plutonium it is possible to measure femtograms (10−15 g) of this element and calculate the isotopic ratios of 238Pu/240Pu and 240Pu/239Pu [3, 7, 8, 10, 15]. The main advantage of AMS compared to conventional MS (TIMS, ICP-MS) is the complete destruction of molecular isobars (e.g. 238UH for 239Pu) by stripping to high positive charge states in the terminal of the tandem accelerator [3, 13, 14]. AMS can minimize the sample size requirements due to its low detection limit of below 106 atom of 239Pu. Further, purification of plutonium leached from the discs after semiconductor alpha spectrometry is not necessary. However, AMS operation is complex and a well experienced team is required. Also, the analysis is quite expensive [16]. Summarizing, the AMS has very short analysis times, high sensitivity and enables the measurement of the distinction of 239Pu and 240Pu and sometimes 241Pu [4, 17]. In spite of these advantages, it is not yet possible to determine 238Pu due to the interferences of 238U [18].
The Baltic Sea is a small shelf sea, very sensitive to human impact and marine plants and animals accumulate radionuclides from the aquatic environment [19–24]. The main source of plutonium in the Baltic Sea and the Poland territory, before the Chernobyl accident, was global fallout from the nuclear weapons testing. Other sources of plutonium, e.g. releases from the spent fuel facilities at Sellafield (UK) and Cap de la Hague (France), are less important. Since 26 April 1986 there has been a new source of plutonium—the Chernobyl accident—which should be taken into consideration in environmental risk assessments [19]. Different sources often exhibit characteristic Pu isotope ratios, and these ratios are known to vary with factors like nuclear fuel burn-up, reactor type and operating history, neutron flux and weapon yield. Hence, these ratios can be used to identify the origin of contamination, calculate inventories [25].
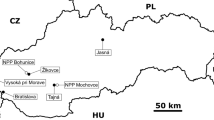
This work presents the results of the 240Pu/239Pu isotope ratios determination in air dust samples collected over Gdynia (Poland) and three Baltic fish species using AMS technique (Fig. 1).
Materials and methods
The atmospheric air dust samples were collected monthly in 1986 over Gdynia (northern Poland) while three species of fish: flounder, herring and cod from the Gulf of Gdańsk (the southern Baltic Sea) were caught in 1997 (Fig. 1). The whole fish were measured and weighed. Organs and tissues were dissected from fresh fish immediately after their delivery to the laboratory; the following tissues and organs were isolated: muscles, skeleton, skin, gills, stomach, intestine, liver, gonads and rest [22, 24]. All samples preparations were carried out in clean, low-level analytical laboratories. The pre-weighted filters with air dust samples as well as isolated fish organs and tissues were weighted and digested using 65 % HNO3 with a 242Pu (5 mBq) spikes added as a yield tracers before the radiochemical analysis. The analysis of plutonium treated as follows: samples mineralization in nitric acid and additionally in HF in the case of air dust samples, separation and purification on ion exchange resins, electrolysis on a steel disc and determination of plutonium 238Pu and 239+240Pu isotopes activities by alpha spectrometry [19, 20, 22, 24, 26]. After radiochemical preparation, the activities of 238Pu and 239+240Pu radionuclides were measured separately by alpha spectrometry (Alpha Analyst S470, Canberra Packard, USA) equipped with 12 PIPS detectors (300 or 450 mm2 area each, FWHM = 18 keV). The accuracy and the precision of the plutonium analysis were satisfactory, i.e., precision less than 7 % (1.5–6.4 %), as estimated by analysis of the International Atomic Energy Agency standard materials (IAEA-367, IAEA-384, IAEA-414). The chemical yield of plutonium analysis varied from 60 to 90 %. The results are also given with standard deviation (SD) calculated for 95 % confidence intervals. The detection limits were calculated at 0.05 mBq for 239+240Pu [27].
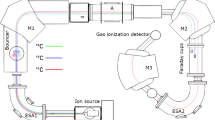
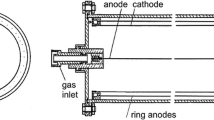
The 240Pu/239Pu atomic ratios were measured in 2007 using 1 MV compact AMS system located at Centro Nacional de Aceleradores (CNA) (Seville, Spain). All sources of air dust and fish samples previously separated, purified and measured 238Pu and 239+240Pu using the alpha spectrometer, were prepared for AMS measurements. The samples (alpha spectrometry targets) were dissolved in 2 M HNO3 and the plutonium fractions were transferred to the solution. After isolating the sample from the disc, 1 mg of iron (i.e. as Fe(NO3)3) was added and then the solution was evaporated in the PTFE tube. The iron was joined with the plutonium and used as the carrier. After evaporating, the Pu/Fe matrix was transferred to the ceramic crucible and baked in 800 °C for 3 h, to convert the nitrate components to oxides. Further 2 mg of aluminum powder was added, to give the samples indispensable electric and thermal conductivity. After that the sample was pressed into the aluminum holder with thin Cu wire and the Al/Cu target cathode was prepared [2, 10, 25, 28, 29]. AMS system at CNA is a 1 MV Tandetron® system is equipped with a carousel for 200 samples and the accelerator. Argon gas is used as a stripper. Finally, the ions are counted with a two-anode gas ionization detector provided with a 75 nm thick 8 × 8 mm2 silicon nitride window (Fig. 2; [2]). The mass or energy resolution of the magnets are 680 and 1,300 mm/(ΔM(E)/M(E)), respectively. At the image of the ESA, whose energy resolution is 1,300 mm/(ΔE/E), a pair of horizontal slits helps as well to control the final energy of the particles arriving at the detector. The software of AMS, designed to support the injection of different masses into the detector by changing the tuning of the system sequentially, allows us to perform automatic measurement of plutonium isotopes. Usually, pulses of 15 s are dedicated to 239Pu, of 30 s to 240Pu and of 5 s to 242Pu. The final results are the 239,240Pu/242Pu atomic ratios. 242Pu is used as the normalization isotope for the measurements and as the radiochemical tracer (previously used in alpha spectrometry analysis) for the chemical procedure in the case of processed environmental samples. A single measurement is completed when this sequence is repeated 5 times, and a real sample analysis comprises usually between five and seven single measurements alternated with standards and blanks. The typical instrumental error of the final ratios is about 1.5 % (standard deviation of the mean). The AMS technique was applied to three reference materials IAEA-381, IAEA-Soil-6 and IAEA-375 and the accuracy and precision were estimated. The IAEA certifies the 239+240Pu activity concentration for all of them, but the 240Pu/239Pu atomic ratio only for the sample 381 while for the IAEA-Soil-6 240Pu/239Pu atomic ratios were referred to the results given by Muramatsu et al. [30]. The accuracy as well as the precision were satisfactory, estimated at less than 5 % for 240Pu/239Pu measurements and less than 2 % for 239+240Pu measurements [2, 29]. The detection limits were achieved at ∼106 atoms for each isotope [10].
However, according to our fish samples, the 239+240Pu contents were too low to measure 240Pu/239Pu atomic ratios in most of analyzed organs and tissues.
Results and discussion
Statistical analysis of the 239+240Pu concentrations as well as the 240Pu/239Pu atomic ratio values in analyzed samples showed that the differences between them were statistically significant, in spite of their uncertainties. The plutonium nuclide compositions in the filter samples corresponded to their means of release into the environment. The measurements of the atmospheric fallout samples collected monthly throughout 1986 allowed the Chernobyl peak effect on Gdynia (Poland) to be observed (Table 1). From January to March 1986 we also observed quite high 240Pu/239Pu atomic ratios (from 0.24 to 0.36) when compared to the global stratospheric fallout 240Pu/239Pu atomic ratios (0.18) [31]. The values of 240Pu/239Pu atomic ratios given for the global stratospheric fallout were 0.176 ± 0.014 and 0.180 ± 0.014, weapon-grade Pu generally presents a very low 240Pu/239Pu atomic ratio varying from 0.02 to 0.06 and those from close-in tropospheric fallout from the Pacific Proving Grounds (PPG) in the Marshall Islands was 0.33–0.36 [2, 4, 31–36]. According to the Polish conditions (East Europe) the average value for the global atmospheric fallout (240Pu/239Pu atomic ratio at 0.18) is more probable. Air filter data measured from January to March 1986 should correspond with typical values for material affected only by the global fallout. One of the reasons for the enhanced 240Pu/239Pu atomic ratio in the air dust over Poland could, for example, have been the Chernobyl-1 accident in September 1982. This time the part of the core reactor was released into the industrial zone and city of Pripyat, causing irradiation of the surroundings [37]. The other, more probable reason is the impact of aerosols from the North Sea inflows. The atomic ratios of 240Pu/239Pu from the western European nuclear fuel reprocessing facilities were 0.34 ± 0.03 in the effluent from Cap de la Hague and they were reported over Poland [38, 39]. Although other accidents should also be recalled (e.g. Kyshtym 1957, Leningrad 1975) the global atmospheric fallout had the main impact on the 240Pu/239Pu atomic ratios [40, 41]. The differences and significant decrease of 240Pu/239Pu atomic ratios as well as 239+240Pu concentrations in January and February 1986 can be explained by very low precipitation in February 1986 caused by continental high-pressure area from Russia which brought very low temperature and lack of snow–the average monthly precipitation over Gdynia in January, February and March 1986 was calculated at 44, 5 and 22 mm and the average monthly temperature in February was −5.5 °C while in January only −0.1 °C. It could mean lower impact of the western nuclear fuel reprocessing facilities with higher 240Pu/239Pu atomic ratios. Weather changes as well as higher temperatures in March 1986 brought more precipitation what influenced on higher 240Pu/239Pu atomic ratio [42].
Obtained data indicated a significant increase in the 240Pu/239Pu atomic ratio from 0.29 ± 0.04 in March 1986 to 0.47 ± 0.02 in April 1986 (Table 1). Our value corresponds with the 240Pu/239Pu atomic ratios calculated for the reactor core (from 0.39 to 0.563) [43, 44]. Such a high 240Pu/239Pu atomic ratio (0.42) was also observed a few days after the Chernobyl accident in aerosols over Vilnius (Lithuania) [45]. From November 1986, the 240Pu/239Pu atomic ratios decreased and had returned to similar level as before the accident [19]. In December 1986 the 240Pu/239Pu atomic ratio was calculated at 0.37 ± 0.10, which is close to the value recorded in January 1986—0.36 ± 0.04. Also 239+240Pu concentration from December 1986 (26.3 ± 5.9 μBq g−1 dw) was similar to those reported for January and February 1986 (32.9 ± 3.2 and 26.2 ± 3.6 μBq g−1 dw respectively). Slightly lower 240Pu/239Pu atomic ratios were found in forest soil from north-eastern Poland a few years later: Szczerba 0.309 ± 0.011, Plaska 0.348 ± 0.011, Trzcianka 0.308 ± 0.024 [46]. Although 239+240Pu concentrations obviously increased, we can clearly expect 240Pu/239Pu atomic ratios to continue falling achieving the values typical for the global atmospheric fallout with an impact of the nuclear fuel reprocessing facilities. Over Vilnius (Lithuania) this ratio decreased from 0.42 in 1986 to 0.20 in 2002 [45].
The measurements of fish samples allowed obtaining only a few results, while most of them were below the detection limit, probably the 239+240Pu content was too low to be measured (Table 2). However, obtained data were much differentiated; the highest 240Pu/239Pu atomic ratio was found in stomach of pelagic plankton-feeding herring (Clupea harengus) (0.44 ± 0.03) and the lowest in liver of pelagic predatory cod (Gadus morhua) (0.055 ± 0.016) (Table 2). High 240Pu/239Pu atomic ratio in herring stomach (0.44 ± 0.03) can be connected to its indirect contact with food and water enriched with the Chernobyl plutonium [23]. Low 240Pu/239Pu atomic ratio in cod liver (0.055 ± 0.016) indicates the liver is not enriched as the other organs of the analyzed fish. Previous studies showed that the highest amount of plutonium in the long-living predatory cod was mainly in intestine and skeleton. The liver probably reflected the plutonium metabolism and its changes in the environment in less extent in comparison to the other organs and tissues and contained mainly the fraction of weapon-grade plutonium [22].
Our previous studies showed the plutonium analyzed in fish skin came mainly from the water as adsorbed by mucus; 66 % of total 239+240Pu in skin was connected to mucus, while only 34 % was inbuilt in the true skin and the skales [23]. Low 240Pu/239Pu atomic ratio in skin of benthic flounder (0.26 ± 0.04) could indicate lower amount of the Chernobyl-derived plutonium in bottom water or could indicate mainly the global atmospheric fallout plutonium with increasing impact of the Chernobyl-derived plutonium. However, the study about determination of plutonium in flora and fauna representatives from the Gulf of Gdańsk in 1987–1991 showed low participation of Chernobyl-derived plutonium and its deposition in sediments (a few percents) and Bivalvia (10 %). Chernobyl plutonium in phytobenthos and fish was not observed at that time [19]. Our later studies carried out in 1997, showed bigger amount of the Chernobyl-derived plutonium in fish from the Gulf of Gdańsk. The highest amounts of the Chernobyl plutonium were found in organs and tissues of pelagic herring and cod (10–80 %) while the lowest in benthic flounder (0–50 %) [22]. Despite all these years from the Chernobyl accident its radiological effects could be still observed in the environment of the southern Baltic caused by the effect of late inflow of plutonium to Baltic Sea increasing the impact of the global atmospheric fallout as well as the nuclear fuel reprocessing facilities.
Conclusions
We estimated the 240Pu/239Pu atomic ratios in the air dust as well as Baltic fish and obtained results were much differentiated. The nuclide compositions of plutonium in the filter samples correspond to their means of production. AMS measurements of atmospheric fallout samples collected monthly throughout 1986 allowed the Chernobyl peak effect on Gdynia (Poland) to be observed. Obtained data indicated a significant increase in the 240Pu/239Pu atomic ratio from 0.29 ± 0.04 in March 1986 to 0.47 ± 0.02 in April 1986 what corresponds with the 240Pu/239Pu atomic ratios calculated for the reactor core (from 0.39 to 0.563). Further studies showed in November 1986 the 240Pu/239Pu atomic ratio decreased. According to fish samples most of the analyzed 240Pu/239Pu atomic ratios were below the detection limit, probably due to low total 239+240Pu content in the analyzed samples. However, obtained results showed the radiological effects of the Chernobyl accident could be still observed in the environment of the southern Baltic caused by the effect of late inflow of plutonium to Baltic Sea increasing the impact of the global atmospheric fallout as well as the nuclear fuel reprocessing facilities.
A good option for alpha spectrometry complement is accelerator mass spectrometry (AMS). AMS is characterized by low measurement background, high detection efficiency and what is the most useful, needs the same radiochemical method as alpha spectrometry which is fully applicable for the accurate determination of the plutonium in different environmental matrixes.
References
Browne E, Firestone RB, Shirley VS (1986) Table of radioactive isotopes. Wiley and Sons, New York
Chamizo E, Enamorado SM, Garcia-Leon M, Suter M, Wacker L (2008) Inst Methods Phys Res B 266:4948
Hrnecek E, Steier P, Wallner A (2005) Appl Radiat Isot 63:633
Wacker L, Chamizo E, Fifield LK, Stocker M, Suter M, Synal HA (2005) Nucl Inst Methods Phys Res B 240:452
Röllin S, Sahli H, Holzer R, Astner M, Burger M (2009) Appl Radiat Isot 67:821
LaMont SP, Glover SE, Filby RH (1998) J Radioanal Nucl Chem 234(1–2):195
Fifield LK, Cresswell RG, Di Tada ML, Ophel TR, Day JP, Clacher AP, King SJ, Priest ND (1996) Nucl Inst Methods Phys Res B 117:295
McAninch JE, Hamilton TF, Brown TA, Jokela TA, Knezovich JP, Ognibene TJ, Proctor ID, Roberts ML, Sideras-Haddad E, Southon JR, Vogel JS (2000) Nucl Inst Methods Phys Res B 172:711
Fifield LK, Synal H-A, Suter M (2004) Nucl Inst Methods Phys Res B 223–224:802
Chamizo E, Garcia-Leon M, Synal H-A, Suter M, Wacker L (2006) Nucl Inst Methods Phys Res 249:768
Wyse EJ, Lee SH, Rosa JL, Povinec P, Mora SJ (2001) J Anal Atom Spectr 16:1107
Hrnecek E, Aldave de las Heras L, Betti M (2002) Radiochim Acta 90:721
Oughton DH, Day P, Fifiled LK (2001) Plutonium measurements using accelerator mass spectrometry: methodology and applications. Elsevier, Amsterdam
Child DP, Hotchkis MAC, Whittle K, Zorko B (2010) Nucl Instr Methods Phys Res B 268:820
Fifield LK (2008) Quat Geochronol 3(3):276
Lee SH, Gastaud J, La Rosa JJ, Liong Wee Kwong L, Povinec P, Wyse E, Fifield LK, Hausladen PA, Di Tada LM, Sanots GM (2001) J Radioanal Nucl Chem 248(3):757
Tims SG, Pan SM, Zhang R, Fifield LK, Wang YP, Gao JH (2010) Nucl Instr Methods Phys Res B 268:1155
Bisinger T, Hippler S, Michel R, Wacker L, Synal H-A (2010) Nucl Instr Methods Phys Res B 268:1269
Skwarzec B (1995) Polon, uran i pluton w ekosystemie południowego Bałtyku. Rozprawy i monografie 6, Instytut Oceanologii PAN 6, Sopot
Skwarzec B (1997) Chem Anal (Warsaw) 42:107
Skwarzec B (1997) Ambio 26:113
Skwarzec B, Strumińska DI, Boryło A (2001) J Environ Radioact 55:167
Struminska DI, Skwarzec B (2004) J Environ Radioact 72:355
Strumińska DI, Skwarzec B (2006) J Radioanal Nucl Chem 268:59
Oughton DH, Skipperud L, Fifield LK, Cresswell RC, Salbu B, Day JP (2004) Appl Radiat Isot 61:249
Skwarzec B (2009) Determination of radionuclides in aquatic environment, analytical measurement in the aquatic environments. CRC, Boca Raton
Strumińska-Parulska DI, Skwarzec B, Fabisiak J (2011) J Environ Radioact 102:1105
Tims SG, Hancock GJ, Wacker L, Fifield LK (2004) Nucl Instr Methods Phys Res B 223–224:796
Chamizo E, Jiménez-Ramos MC, Wacker L, Vioque I, Calleja A, García-León M, García-Tenorio R (2008) Anal Chim Acta 606:239
Muramatsu Y, Yoshida S, Tagami K, Uchida S, Rtihm W (2001) Radioact Environ 1:63–77
Agarande M, Benzoubir S, Neiva-Marques AM, Bouisset P (2004) J Environ Radioact 72:169
Buesseler KO (1997) J Environ Radioact 36(1):69
Diamond H, Fields PR, Stevens CS, Studier MH, Fried SM, Inghram MG, Hess DC, Pyle GL, Mech JF, Manning WM, Ghiorso A, Thompson SG, Higgins GH, Seaborg GT, Browne CI, Smith HL, Spence RW (1960) Phys Rev 119:2000
Krey PW, Hardy EP, Pachucki C, Rourke F, Coluzza J, Benson WK (1976) Mass isotopic composition of global fallout plutonium in soil, Transuranium Nuclides in the Environment. IAEA-SM-199-39, Vienna
Komura K, Sakanoue M, Yamamoto M (1984) Health Phys 46:1213
Kelley JM, Bond LA, Beasley TM (1999) Sci Total Environ 237–238:483
Medvedev G (1990) Nucleonics week (US), 31 May 1990
Oughton DH, Skipperud L, Salbu B, Fifield LK, Cresswell RC, Day JP (1999) Marine pollution. IAEA-SM-354-59, Vienna
Kierepko R, Mietelski JW (2010) Nukleonika 55(2):201
Bojarski C, Jaśkowski J, Miłkowski W, Piękoś R, Stępczak K (1992) Katastrofa w czarnobylu a polska. Raport. Wydawnictwo Gdańskie, Gdańsk
IAEA–International Atomic Energy Agency (1993) A critical documentation of the agency’s policy. Hannover, Vienna
IMGW (1987) Warunki środowiskowe polskiej strefy południowego Bałtyku w 1986 roku, Materiały IMGW, Gdynia (http://baltyk.imgw.gdynia.pl/warunki/1986.html). Accessed 23 Feb 2013
Kirchner G, Noack CC (1988) Nucl Safety 29:1
Begichev SN, Borovoj AA, Burlakov EV, Gagarinsky AJ, Demin VF, Khrulev AA, Khodakovsky LL (1990) Fission product transport processes in reactor accidents. Hemisphere Publishing Co., New York
Lujaniene G, Aninkevicius V, Lujanas V (2009) J Environ Radioact 100:108
Ketterer ME, Hafer KM, Mietelski JW (2004) J Environ Radioact 73:183
Acknowledgments
The author would like to thank the Ministry of Science and Higher Education for the financial support under grant DS/530-8120-D196-13 and Elena Chamizo (CNA, Spain) for her help in the measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Strumińska-Parulska, D.I. Accelerator mass spectrometry (AMS) in plutonium analysis. J Radioanal Nucl Chem 298, 593–598 (2013). https://doi.org/10.1007/s10967-013-2448-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2448-4