Abstract
Ion exchange is a simple and efficient method for separating no-carrier-added 64Cu from an irradiated Ni target. We developed a semi-automated two-round 64Cu separation system equipped with a strong-base anion exchange resin column. We first verified the efficiency of the system using a non-radioactive substitute consisting of 25 mg of Ni and 127 ng of Cu, and confirmed that Cu was completely eluted at the second round of the separation step. After the bombardment, separation of 64Cu from the Ni target was achieved with high radiochemical purity. 64Cu produced and separated in this study had an extremely low level of Ni impurity. It could be used for labeling monoclonal antibodies for antibody positron emission tomography imaging and synthesizing radiopharmaceuticals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
64Cu is a useful radionuclide for positron emission tomography (PET) [1, 2] as well as a potential radiation therapeutic reagent [3, 4], due to its intermediate half-life of 12.7 h and emission characteristics of both β− (40 %) and β+ (19 %). 64Cu is normally produced from highly enriched 64Ni via the reaction of 64Ni (p, n) 64Cu by a cyclotron [5, 6]. For the separation of 64Cu from a 64Ni target and other trace amounts of byproducts, several methods can be used, such as precipitation, solvent extraction, electroplating, and ion exchange [7–9]. Among them, an ion exchange methodology using strong-base anion exchange resin is the most effective for the separation and purification of 64Cu [5, 10–12]. However, it is difficult to completely separate a tiny fraction of the cyclotron-produced 64Cu from the extremely large amount of the 64Ni target. For example, the ratio of 64Ni target to 64Cu is in the order of millions.
In the case of handling cyclotron-produced radioactive nuclides, we must avoid any manual performance, as this involves very high irradiation doses to the operators. Obata et al. [13] developed a remote-controlled 64Cu-separation apparatus equipped with a strong-base anion exchange resin column. In this study, we developed a semi-automated 64Cu-separation system, which is placed in the hot cell. It enabled the separation of high-quality and no-carrier-added 64Cu suitable for labeling monoclonal antibodies for antibody PET imaging.
Materials and methods
Reagent
Isotopically enriched 64Ni (99 %) was purchased from Isoflex Co. (San Francisco, CA, USA). Ultra-grade HCl and HNO3 were purchased from Sigma Aldrich (Tokyo, Japan). Cu and Ni standard solution (1 mg/ml) for atomic absorption spectrometry were obtained from Wako Pure Chemical Industries (Tokyo, Japan). Ultra-pure water was also from Wako Pure Chemical Industries.
Preparation of Ni target and 64Cu production
The Ni target was prepared by the electrodeposition of enriched 64Ni on a 31-mm-diameter Au disk (Sumitomo Heavy Industries, Ltd., Tokyo, Japan). The Au disk, with the plated 64Ni (0.5 cm2), was mounted on a water-cooled target holder and irradiated with 12 MeV protons using a biomedical cyclotron (Cypris HM-12S, Sumitomo Heavy Industries, Ltd.). The production of 64Cu was performed at currents of 15–20 μA.
Separation of 64Cu
After bombardment, 64Cu was separated from the Ni target in a single step on a strong-base anion exchange resin column using a prototype semi-automated separation apparatus (Sumitomo Heavy Industries, Ltd.). All of the solution was pumped and supplied to the column by N2 gas. The irradiated 64Ni was dissolved off the Au disk in 10 ml of 6 M HCl at 200 °C for 40 min and evaporated to dryness. The residue was dissolved in 10 ml of 6 M HCl and transferred onto a 0.8 × 4-cm AG1-X8 anion exchange column (Bio-Rad Laboratories, Inc., Hercules, CA, USA) equilibrated with 6 M HCl. The column was washed twice with 8 and 5 ml of 6 M HCl, and we collected 64Ni effluent for recycling. After switching the eluent to 10 ml of 0.1 M HCl, 64Cu was eluted and collected. 64Cu radioactivity of each eluate was measured in a dose calibrator (CRC-25PET, Capintec, Inc., Ramsey, NJ, USA).
Non-radioisotope substitute for target-dissolved solution
The solution consisting of non-radioactive Ni and Cu was prepared to substitute for radioactive target-dissolved solution. A 101 mg of nickel chloride hexahydrate (25 mg of Ni) and 341 ng of copper chloride dihydrate (127 ng of Cu) were dissolved in 10 ml of ultra-grade nitric acid. The amount of Ni and Cu was described in a previous report [14].
Atomic absorption spectrometry
A flame atomic absorption spectrometer (Z-9000, Hitachi, Ltd., Tokyo, Japan) equipped with a hollow cathode lamp was used for the determination of Ni and Cu. The wavelengths were 232.0 and 324.8 nm for Ni and Cu, respectively. Analytical working solution containing 100, 200, 400, and 800 ng of Ni and 12.5, 25, 50, and 100 ng of Cu were prepared by the appropriate dilution of the 1 mg/ml standard solution with ultra-grade nitric acid, respectively. The absorbance of blank, analytical solutions, and sample solutions was measured successively at the optimized operating conditions.
Determination of radionuclide purity
The determination of radionuclide purity of separated 64Cu was performed by γ-spectrometry with a Ge semiconductor detector (GMX15P4-70, SEIKO EG&G, Tokyo, Japan). Data analysis was performed using Gamma Studio software (SEIKO EG&G).
Results and discussion
Table 1 shows the results of two independent production of 64Cu. The separation of 64Cu from the Ni target was performed in a single-round anion exchange resin column using a prototype semi-automated apparatus. Nearly 50 % of 64Cu was eluted and separated. However, a significant amount of the 64Cu radioisotope went to Ni waste (column flow-through and effluent) and remained on the resin column. The results showed the poor reproducibility of the single-round separation methodology.
In order to investigate the separation efficiency of the single-round methodology, a non-radioisotope substitute consisting of 25 mg of Ni and 127 ng of Cu was applied to the prototype apparatus. A 1-ml fraction was collected at each step for Ni or Cu determination using the flame atomic absorption spectrometer. As shown in Fig. 1, Ni was not completely washed out even in the last washing step with 6 M HCl. A large amount of Ni remained on the column. Ni was continuously stripped from the column and mixed in the Cu eluate portion. Cu was efficiently eluted with 1 M HCl compared with 0.1 M HCl. However, twice the amount of Ni (ab. 200 ng) was contaminated even in the second 1-ml portion of the Cu eluate (ab. 100 ng).
Insufficient separation performance by the prototype separation apparatus. Non-radioisotope substitute solution consisting of Ni and Cu was applied to the single-round prototype separation apparatus. Cu was eluted with 0.1 M HCl (a) or 1 M HCl (b) solution. A 1-ml fraction was collected at each step, and the Ni and Cu concentration was determined by the atomic absorption spectrometer
Because of the insufficient separation performance of the single-round methodology, we developed the two-round separation methodology and constructed a new semi-automated separation apparatus (Figs. 2, 3). After the first round of column treatment, eluted and collected Cu in 1 M HCl solution was adjusted to 6 M HCl solution by adding an appropriate volume of 12 M HCl. It was further purified through the second round of column treatment (Fig. 2). All the solution was transferred to the column reservoir by N2 gas. Then, the solution was passed through the resin column from the reservoir at a normal atmospheric pressure (Fig. 3). We analyzed the Cu separation performance by this apparatus using a non-radioisotope substitute. A significant amount of Ni was still co-eluted with Cu at the first round of separation. However, by the second round of separation, a negligible amount of Ni was found in the Cu eluate (Fig. 4). Cu was completely eluted in the second to fourth fractions.
Two-round 64Cu separation system. (1) Vessel for target dissolving, (2) 6 M HCl washing solution vessel, (3) 1 M HCl washing solution vessel, (4) strong-base anion exchange resin column, (5) reservoir, (6) Ni collection vial (column flow-through), (7) vial for effluent, (8) vial for 64Cu eluate 1 (first round), (9) vial for 64Cu eluate 2 (second round)
Separation and purification of Cu from Ni in a non-radioactive substitute. Non-radioactive substitute solution was applied to the two-round semi-automated separation system. A 1-ml fraction was collected at each step, and the Ni and Cu concentration was determined by the atomic absorption spectrometer. a First round of separation, b second (final) round of separation
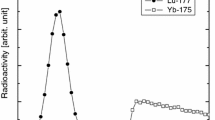
We performed three independent 64Cu separations from a Ni target using the two-round semi-automated separation system. As shown in Table 2, 64–67 % of 64Cu was eluted by the first round of the separation step. In the subsequent second round of separation, 62–66 % of 64Cu was eluted and separated. This revealed that nearly 95 % of 64Cu eluted in the first round of separation was recovered in the second round of separation. A small amount of 64Cu was found in the column flow-through solution and effluent. A negligible amount of 64Cu remained on the resin column. With the use of the Ge detector, the radiochemical purity of the separated 64Cu was confirmed (Fig. 5).
Ion exchange is a simple and efficient method for separating metals. It is based on the formation of anionic metal chloro complexes in a highly concentrated HCl solution and on the difference of their distribution coefficients in a strong-base anion exchange resin [15]. Ni cannot form a chloro complex in HCl solution and is not retained by the anion exchange resin. On the other hand, Cu can form a stable chloro complex in a concentrated HCl solution and is retained by the resin. After Ni is stripped from the resin column with a concentrated HCl solution, Cu can be successfully eluted from the resin column by an appropriate concentration of HCl. 64Cu is produced from highly enriched 64Ni via reactions of 64Ni (p, n) 64Cu by a cyclotron. Because the produced 64Cu is a very tiny fraction of the irradiation Ni target, it is usually difficult to obtain a high yield of 64Cu with extremely low Ni impurity. The remaining Ni will interfere with the antibody-labeling process of 64Cu. It may also be harmful to human health [16, 17].
Taken together, our semi-automated system enabled the separation of high-quality 64Cu suitable for labeling monoclonal antibodies for antibody PET imaging. In the current system, however, the target recovery at the end of bombardment and transfer to the hot cell has to be manually performed. A full-automated target handling system is currently being constructed to reduce exposure doses to the operators to as low as possible.
Conclusion
In this study, we developed a semi-automated two-round 64Cu separation system. It is equipped with a strong-base anion exchange resin column. We first verified the efficient performance of the system, and confirmed that Cu was completely eluted at the second round of the separation step. There was a negligible amount of Ni in the Cu eluate. After the bombardment, separation of 64Cu from the Ni target was successfully achieved with high radiochemical purity. The semi-automated system enabled the separation of cyclotron-produced 64Cu suitable for labeling monoclonal antibodies for antibody PET imaging.
References
Matarrese M, Bedeschi P, Scardaoni R, Sudati F, Savi A, Pepe A, Masiello V, Todde S, Gianolli L, Messa C, Fazio F (2010) Automated production of copper radioisotopes and preparation of high specific activity [64Cu] Cu-ATSM for PET studies. Appl Radiat Isot 68:5–13
Achmad A, Hanaoka H, Yoshioka H, Yamamoto S, Tominaga H, Araki T, Ohshima Y, Oriuchi N, Endo K (2012) Predicting cetuximab accumulation in KRAS wild-type and KRAS mutant colorectal cancer using 64Cu-labeled cetuximab positron emission tomography. Cancer Sci 103:600–605
Connett JM, Anderson CJ, Guo LW, Schwarz SW, Zinn KR, Rogers BE, Siegel BA, Philpott GW, Welch MJ (1996) Radioimmunotherapy with a 64Cu-labeled monoclonal antibody: a comparison with 67Cu. Proc Natl Acad Sci USA 93:6814–6818
Nguyen K, Parry JJ, Rogers BE, Anderson CJ (2011) Evaluation of copper-64-labeled somatostatin agonists and antagonist in SSTr2-transfected cell lines that are positive and negative for p53: implications for cancer therapy. Nucl Med Biol 39:187–197
Zweit J, Smith AM, Downey S, Sharma HL (1991) Excitation functions for deuteron induced reactions in natural nickel: production of no-carrier-added 64Cu from enriched 64Ni targets for positron emission tomography. Appl Radiat Isot 42:193–197
Szelecsényi F, Blessing G, Qaim SM (1993) Excitation functions of proton induced nuclear reactions on enriched 61Ni and 64Ni: possibility of production of no-carrier-added 61Cu and 64Cu at a small cyclotron. Appl Radiat Isot 44:575–580
Tolmachev V, Lundqvist H, Einarsson L (1998) Production of 61Cu from a natural nickel target. Appl Radiat Isot 49:78–81
Shaw MJ, Haddad PR (2004) The determination of trace metal pollutants in environmental matrices using ion chromatography. Environ Int 30:403–431
Fan X, Parker DJ, Smith MD, Ingram A, Yang S, Seville JP (2006) A simple and selective method for the separation of Cu radioisotopes from nickel. Nucl Med Biol 33:938–944
McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ (1997) Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 24:35–42
Hou X, Jacobsen U, Jørgensen JC (2002) Separation of no-carrier-added 64Cu from a proton irradiated 64Ni enriched nickel target. Appl Radiat Isot 57:773–777
Avila-Rodriguez MA, Nye JA, Nickles RJ (2007) Simultaneous production of high specific activity 64Cu and 61Co with 11.4 MeV protons on enriched 64Ni nuclei. Appl Radiat Isot 65:1115–1120
Obata A, Kasamatsu S, McCarthy DW, Welch MJ, Saji H, Yonekura Y, Fujibayashi Y (2003) Production of therapeutic quantities of 64Cu using a 12 MeV cyclotron. Nucl Med Biol 30:535–539
Tazawa S, Hasegawa K, Takahashi K, Yano T, Watanabe Y (2011) Current practice of producing 64Cu-DOTA-Trastuzumab injections RIKEN CMIS according to GMP for investigational products (in Japanese). Pharm Technol Jpn 27:431–440
Michaelis C, Tarlano NS, Clune J, Yolles R (1962) A complete separation of a mixture of iron(III), cobalt(II), molybdenum(VI), aluminum(III), and nickel(II) by ion exchange chromatography. Anal Chem 34:1425–1426
Oller AR, Costa M, Oberdörster G (1997) Carcinogenicity assessment of selected nickel compounds. Toxicol Appl Pharmacol 143:152–166
Zoroddu MA, Schinocca L, Kowalik-Jankowska T, Kozlowski H, Salnikow K, Costa M (2002) Molecular mechanisms in nickel carcinogenesis: modeling Ni(II) binding site in histone H4. Environ Health Perspect 110(Suppl 5):719–723
Acknowledgments
We thank Mr. H. Okamoto and Mr. S. Iwasa (Central Research Laboratory, Okayama University Medical School) for atomic absorption spectrometry. We also thank Dr. T. Nagamatsu (Advanced Science Research Center, Okayama University) for excellent technical assistance and Mrs. T. Terada for preparation of the manuscript. This work was supported in part by Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Toyota, T., Hanafusa, T., Oda, T. et al. A purification system for 64Cu produced by a biomedical cyclotron for antibody PET imaging. J Radioanal Nucl Chem 298, 295–300 (2013). https://doi.org/10.1007/s10967-012-2340-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2340-7