Abstract
In the present work, results of γ-irradiation on normal and functionalized SBA-15 by aurintricarboxylic acid have been reported. Characterization of normal and functionalized SBA-15 particles before and after γ-irradiation was carried out using Fourier-transform infrared technique. Aurintricarboxylic acid ligand connected to SBA-15 was also analyzed using UV/Vis spectrophotometer. The modified sorbent was then used as a new sorbent for separation of trace amounts of praseodymium and lutetium ions from nuclear waste waters in batch techniques. Based on the results of distribution coefficients determination, and investigation of sorption process in various conditions, the parameters were optimized for separation lanthanides. It can be concluded that the functionalized SBA-15 is a promising sorbent for praseodymium and lutetium cations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, nanostructure mesoporous materials have attracted much attention for development of catalytic and adsorption systems. Mesoporous materials have often been functionalized by adding noble ligands to improve their physical and chemical properties [1–3]. Santa Barbara Amorphous (SBA-15) is a highly ordered material possessing a regular two dimensional array of channels. It is similar in structure to the better known MCM-41, however, due to its larger pore size and thicker pore walls it has the ability to capture larger molecules and chemical complexes and has also greater stability in aqueous solutions [4]. This mesoporous material typically has a pore diameter in the order of 7–10 nm, which is twice that of MCM-41 [5].
We used the functionalized SBA-15 as a sorbent for removal of some lanthanides from wastes, because of its promising abilities. So, for evaluation the stability of functional group of aurintricarboxylic acid on the SBA-15 the sorbent was irradiated.
High-level radioactive waste generated by commercial reprocessing facilities contains cesium, strontium, lanthanides, actinides, and other elements [6]. Long-lived radioactive lanthanide species are present at very low concentrations in wastes and the radioactivity of lanthanides is low. Selective removal of these species could greatly reduce the volume of materials for long-term storage. The adsorbent must be selective for the target species among other interfering cations and/or competing ligands. Rare earth cations are hard Lewis acids. These cations are considerably larger than the typical transition-metal cations. Thus both hard ness of anionic ligands and ligand synergy are important attributes in designing effective complex agents for these species. The aurintricarboxylic acid ligand has been synthesized and loaded as monolayer on an extended family of highly ordred mesoporous silicates such as Santa Barbara Amorphous (SBA) [7, 8]. These silicates have been developed as high-performance sorbent materials whose functional groups can be tailored to selectively sequester many target species. The high surface area of the nanopore support coupled with the dense monolayer coating provides high binding capacity and the rigid open pore structure allows for rapid sorption kinetics [9]. The lanthanides are considered only slightly toxic in the Hodge-Sterner classification system and are safely handled with ordinary care. The effect of atomic weight of rare-earth elements on lethality is difficult to assess, but the medium rare-earth elements appear to have a lesser toxicity than light or heavy rare-earth elements [10].
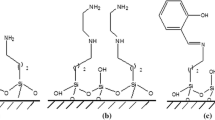
The presented research investigates the normal SBA-15 and functionalized SBA-15 mesopore with aurintricarboxylic acid after γ-irradiation by Fourier-transform infrared spectroscopy and describes a novel functionalized SBA-15 which was used for sorption of praseodymium and lutetium ions from nuclear waste waters in batch (static) techniques. The sorbent can be shown schematically as follows: 
Materials and methods
All reagents used were of analytical grade. Standard solution of lanthanides was prepared by ICP- Standard solution grade.
In this study SBA-15 was synthesized using a triblock copolymer-poly (ethylene glycol)-block-poly (propylene glycol)-block-poly (ethylene glycol) -as a templating agent and coated with aurintricarboxylic acid by contacting the mesoporous silicate with its solution. Normal and functionalized SBA-15 mesopore were then exposed to 60Co γ-irradiation ranging from 1 kGy to a cumulative dose of up to 15 kGy.
For this study, about 50 g of SBA-15 powders was mixed with 188 mL of molar aurintricarboxylic acid solution in a 250 mL polypropylene bottle and agitated for 3 days. After being allowed to settle for 24 h, the clear supernatant solution was discarded and the residue was collected by vacuum filtration through a 0.45 μm nylon membrane. The solid residue was returned to the treatment bottle and agitated with 200 mL of double-distilled water for 24 h to remove the excess of aurintricarboxylic acid. The precipitate was washed and collected by vacuum filtration, and the SBA-15 functionalized by aurintricarboxylic acid ligand was dried at room temperature.
The FTIR spectra of irradiated samples were also studied using KBr (0.01 g powder + 0.5 g KBr) pellet technique in the range of 500–4000 cm−1.
To evaluate the efficiency of sorbent for separation of trace amounts of lanthanides from waste waters distribution coefficient (K d) was determined by exposing the functionalized mesopore SBA-15 to solutions containing lanthanides in definite concentrations ranging from 1 ppb to 300 ppm. The method was used to evaluate the absorption potential of sorbent under batch operation.
For this purpose, 0.2 g of functionalized SBA-15 was mixed with 50 mL of solutions containing lanthanides at 25 °C for 2–70 min. The liquid phase was separated by filtrations concentration of lanthanides in the liquid phase was determined by an inductively coupled plasma- atomic emission spectrometer (ICP-AES, Liberty 150 AX Turbo) and ultraviolet visible spectrophotometer (UV/Vis, hp- 8453). The effect of some parameters such as amount of functionalized mesopore, concentration of lanthanides, and shaking time were investigated by determination of Kd and percent of sorption in various conditions.
Results and discussion
The surface modified SBA-15 was loaded with a chelating agent and assessed for its potential as a highly metal selective adsorbent material for industrial and analytical laboratory applications.
The effect of γ-irradiation on normal and functionalized SBA-15 mesopore before and after γ-irradiation was investigated (with up to 15 kGy dose). FTIR spectra of normal and surface functionalized SBA-15 is shown in Fig. 1.
The results of experiments showed that normal and aurintricarboxylic acid functionalized SBA-15 mesopore remained unchanged up to 15 kGy and it is suitable for applying in lanthanides separation [11].
FTIR spectrum of functionalized SBA-15 was significantly influenced by the underlying silicate surface, whilst the aurintricarboxylic acid coating produces lower transmittance values and new peaks in the Fourier-transform infrared spectrum at higher wave numbers. According to the results of transmission electron microscope (TEM) of SBA-15 pore size distribution was around 25 nm.
UV-Vis absorption spectrum of aurintricarboxylic acid ligand is shown in Fig. 2. Maximum absorption of this ligand is appeared in wavelengths of 207 and 310 nm.
The distribution coefficient (K d) was calculated using the following equation:
where C i and C f are the initial and the final concentration of target ion respectively, m is the mass of functionalized SBA-15(g) and V is the volume of lanthanides solution (mL). There are some factors which can affect the sorption of praseodymium and lutetium by functionalized SBA-15, for this reason, the operating conditions should be optimized. For this purpose, the one-variable-at-a-time optimization was used.
The results of distribution coefficients of different Lanthanides with concentration of 100 ppm are shown in Fig. 3. The main criterion for selection of functionalized SBA-15 as a prospective sorbent is their higher distribution coefficients.
According to Figs. 4, 5, 6, 10 g of modified mesopore is the best value of sorbent in its different concentrations.
Extraction time is one of the most important factors in the most of the extraction methods; The effect of contact time of praseodymium and lutetium with functionalized SBA-15 was examined in the range of 2–30 min in constant experimental conditions (Figs. 7 and 8).
The results show that 12–30 min is best time for this purpose. Thereby, achieve the equilibrium state, is approximately fast. The distribution coefficients of praseodymium and lutetium in different concentrations on the functionalized SBA-15 mesopore have shown in Figs. 9 and 10.
The distribution coefficients decreased by increasing concentration of praseodymium and lutetium ions.
Conclusion
FTIR spectra of normal and surface functionalized SBA-15 revealed that sorbent remained unchanged by γ-irradiation, so, it can be promising sorbent to remove radioactive ions.
Based on the results, the selectivity of functionalized SBA-15 decreased with increasing concentration of praseodymium and lutetium ions. This can be considered as a result of the nature of K d formula, which implies that the sorbent shows higher affinity to the praseodymium and lutetium ions at lower concentration. Subnanomolar concentrations of praseodymium and lutetium can be removed and measured in a short time with a desirable efficiency; so, functionalized SBA-15 can be used to develop a simple and extremely sensitive method for rapid analysis of praseodymium and lutetium ions in waste water, where removal of trace concentration is a big deal for scientists working in the field of liquid waste management. It was not found any other patented or published experiences regarding to praseodymium and lutetium sorption on the SBA-15 grafted with aurintricarboxylic acid ligand worldwide. Further investigation is required to determine their uptake behavior in a dynamic flow conditions. In this concern, it is necessary to consider the effect of some other parameters such as media pH, reaction temperature, stream flow rate for column test, etc.
References
Kageyama K, Tamazawa J, Aida T (1999) Science 285:2113
On DT, Desplantier-Giscard D, Danumah C, Kaliaguine S (2001) Appl Catal A 222:299
Cai W, Zhang Y, Jia J, Zhang L (1998) Appl Phys Lett 73:2709
Vinu A, Hartmann M (2004) Chem Lett 33(5):588
Nguyen S (2003) Master thesis, University of Melbourne, 130
Ohtsuka H, Suzuki K (2005) Proc. Global 2005, Tsukuba, Japan, Oct. 9–13, 499
Geckeler KE, Konstantin V (1996) J Environ Sci Technol 30:725
Romanovki VV, white D, Xu J, Hoffman DC, Raymond KN (1999) Solvent Extr Ion Exch 17:55
Lin WY, Fryxell GE, Busche BJ, Birnbaum JC (2003) Sep Sci Technol 38:3809
Haley TJ, Gschneider KA, Eyring L (eds) (1979) Handbook on the physics and chemistry of the rare earths, vol 4. North Holland Publ. Co, Amsterdam, the Netherlands, p 40
Kikuchi T, Goto I, Suzuki K (2006) J Nucl Sci Technol 43:690
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mallah, M.H., Ghannadi Maragheh, M., Badiei, A. et al. Novel functionalized mesopore of SBA-15 as prospective sorbent for praseodymium and lutetium. J Radioanal Nucl Chem 283, 597–601 (2010). https://doi.org/10.1007/s10967-010-0452-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0452-5