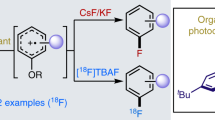
Abstract
For the radiofluorination of benzenes and benzene derivatives, the electrophilic reaction with [18F]F2 is a very common route. Yet, aromatic nucleophilic substitution (SNAr) by n.c.a [18F]fluoride, which can be produced efficiently in high amounts, has been considered to be very desirable. However, to facilitate 18F-labelling via SNAr at an electron rich aromatic system, an appropriate leaving group must be present together with an auxiliary group in ortho or para position to the leaving group. An interesting alternative for the auxiliary group is the heteroatom of a heteroaromatic system, for which pyridine is a leading example. Dolci et al. (J Label Compd Radiopharm 42:975–985, 1999) have evaluated the scope of the nucleophilic aromatic fluorination of 2-substituted pyridine rings using the activated K [18F]F-K222 complex. As methyl and methoxy groups are known to enhance the electron density of an aromatic system by the +I and the +M effect, respectively, SNAr is unlikely to occur. Until now, the effect of these substituents has not been studied towards the 18F-radiofluorination of substituted 2-nitropyridines by use of [18F]fluoride. Therefore, we have investigated the effect of methoxy and methyl groups in 2-nitropyridines. The results showed that 3-methoxy-2-nitropyridine and 3-methyl-2-nitropyridine can efficiently be substituted by [18F]fluoride with high RCY’s (70–89%) in short reaction times (1–30 min) at a reaction temperature of 140 °C. Moreover, 3-methoxy-6-methyl-2-[18F]fluoropyridine was obtained from the corresponding nitro-precursor in a high yield of 81 ± 1% after 30 min at 140 °C. In case of 2-nitropyridines data indicates the effect of methyl and methoxy groups on SNAr to be of minor importance.



Similar content being viewed by others
References
Ding YS, Shiue CY, Fowler JS, Wolf AP, Plenevaux A (1990) J Fluorine Chem 48:189–205
Rengan R, Chakraborty PK, Kilbourn MR (1993) J Label Compd Radiopharm 33(7):563–572
Shen B, Löffler D, Zeller KP, Reischl G, Machulla HJ, Zeller KP (2009) J Fluorine Chem 130(2):216–224
Shen B, Löffler D, Zeller KP, Ubele M, Reischl G, Machulla HJ (2007) Int J Appl Radiat Isot 65(11):1227–1231
Shen B, Löffler D, Zeller KP, Ubele M, Reischl G, Zeller KP, Machulla HJ (2007) J Nucl Med 48(suppl 2):317P
Irie T, Fukushi K, Ido T (1982) Int J Appl Radiat Isot 33:445–448
Knust EJ, Müller-Platz C, Schüller M (1982) J Radioanal Nucl Chem 74:283–291
Dolci L, Dollé F, Jubeau S, Vaufrey F, Crouzel C (1999) J Label Compd Radiopharm 42:975–985
Mongin F, Trécourt F, Mongin O, Quéguiner G (2002) Tetrahedron 58(2):309–314
Shen B (2008) Dissertation. http://tobias-lib.ub.uni-tuebingen.de/volltexte/2008/3666/pdf/doktorarbeit_final_081219.pdf
Breitmaier E, Voelter W (1987) Carbon-13 nmr spectroscopy-high resolution methods and applications in organic chemistry and biochemistry. VCH Verlagsgesellschaft GmbH, Weinheim, pp 135, 184–185
Acknowledgments
The project was supported by funding of Deutsche Forschungsgemeinschaft (DFG) with the grant no. MA 1096/8-1. Moreover, the authors are particularly grateful to Prof. Dr. S. Reske for allowing to continue and finish the project together with giving continuous encouragement and stimulation throughout this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malik, N., Solbach, C., Voelter, W. et al. Nucleophilic aromatic substitution by [18F]fluoride at substituted 2-nitropyridines. J Radioanal Nucl Chem 283, 757–764 (2010). https://doi.org/10.1007/s10967-009-0410-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0410-2