Summary
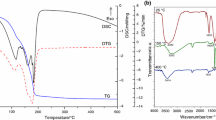
The thermal decomposition of manganese tris(malonato)ferrate(III) hexahydrate, Mn3[Fe(CH2C2O4)3]2 . 6H2O has been investigated from ambient temperature to 600 °C in static air atmosphere using various physico-chemical techniques, i.e., simultaneous TG-DTG-DSC, XRD, Mössbauer and IR spectroscopic techniques. Nano-particles of manganese ferrite, MnFe2O4, have been obtained as a result of solid-state reaction between a-Fe2O3 and MnO (intermediate species formed during thermolysis) at a temperature much lower than that for ceramic method. SEM analysis of final thermolysis product reveals the formation of monodisperse manganese ferrite nanoparticles with an average particle size of 35 nm. Magnetic studies show that these particles have a saturation magnetization of 1861G and Curie temperature of 300 °C. Lower magnitude of these parameters as compared to the bulk values is attributed to their smaller particle size.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Randhawa, B., Kaur, M. & Gandotra, K. Mössbauer studies on the thermolysis of manganese tris(malonato)ferrate(III)hexahydrate. J Radioanal Nucl Chem 269, 69–74 (2006). https://doi.org/10.1007/s10967-006-0231-5
Issue Date:
DOI: https://doi.org/10.1007/s10967-006-0231-5