Abstract
Two castor oil / poly (N-vinyl-2-pyrrolidone) / ammonium persulfate (CAO/PVP/APS) hybrids were synthesized via reaction of castor oil with PVP of molecular weights 40000 and 1300000 Dalton in presence of APS as an initiator. The optimum reaction conditions are: PVP/CAO weight ratio, 15%; PVP concentration, 60%; APS conc., 4% (based on weight of CAO); reaction temperature, 80 °C; reaction time, 60 min. The synthesized CAO/PVP/APS hybrids were characterized via FTIR analysis whereas their emulsions particle sizes were determined using TEM analysis. Incorporation of either of the aforementioned hybrids emulsions in the easy care finishing formulations of cotton fabric results in promoting of fabric softness, tensile strength, flexibility, and antibacterial activities of treated fabric along with reducing fabric wettabiliy and resiliency. Inclusion of Ag-NPs in the aforementioned finishing bath significantly improves extent of the antibacterial activities of treated fabric. The treated fabric morphology was examined using SEM as well as EDX analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Textile finishing is usually performed as a last step to impart a textile material with functional properties such as water and oil repellency [1,2,3,4,5], resistance to crease [6,7,8], resistance to microbes [9, 10], protection from UV radiation [11, 12], self-cleaning [13], pleasant scent [14], hand building [15], and softness [16].
Textile softener is a finishing agent improves the textile handle giving rise to a pleasing touch upon applying it to the textile material. In general, the textile softener is a lubricating agent that facilitates fiber slippage inside the fabric structure. Textile softeners can be broadly classified into five main types according to their chemical structure that are silicone, cationic, anionic, nonionic, and amphoteric softeners. A wide range of finishing agents is available in the market to satisfy the consumer’s demands [16,17,18,19]. The textile finishing future trend is developing textiles to have multifunctional properties that are efficient, cost effective, durable, and safe for both the consumer and the environment [10, 12, 13, 16].
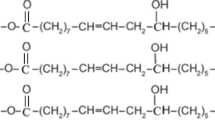
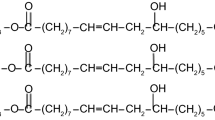
Nowadays, researchers are looking for plant oils such as castor oil, soybean oil, olive oil, and linseed oil as renewable resources for using in various industries such as cosmetics, lubricants, paints, coats, etc. [1, 20,21,22,23]. Among the plant oils, castor oil occupies unique standing due to its chemical structure containing double bonds, hydroxyl groups, and carboxyl groups of the ricinoleic acid (Fig. 1) [1, 20] which is the main component of castor oil in addition to linoleic, oleic, and stearic acids. The chemical structure of castor oil offers a wide range of applications including lubricants, varnishes, paints, inks, cosmetics, plastics, textile dyes, and soaps [1, 20,21,22,23].
Chemical structure of castor oil [20]
Emulsions of castor oil are commonly used in cosmetics, pharmaceutical products, and food industries. The castor oil emulsions can deliver the hydrophilic and lipophilic drugs [24]. Castor oil can be emulsified by means the normal emulsifiers in addition to many gums such as Arabic gum as well as Terminalia gum [25, 26]. A formula consists of polyoxyethylene sorbitan monostearate and sorbitan monooleate can emulsify castor oil as reported by Zhang et al. [27].
In the textile field, many textile auxiliaries were synthesized using castor oil as a raw material. The Turkey red oil which is the sulphated castor oil was used as an anionic textile softener or wetting agent depending on the sulphation degree of castor oil [28]. Nair et al. utilized the coconut Oil, vegetable Ghee, and castor oil to produce eco friendly textile softeners [29]. A new textile softener was synthesized by the thermal treatment for a mixture of poly (N-vinyl-2-pyrrolidone) (PVP) and castor oil at 150 °C for 1 h [30]. Castor oil based water repellent agent was prepared using 2,4-toluene di-isocyanate as a crosslinker linker followed by emulsification the product with stearic acid/triethanol amine emulsifying system [1].
On the other hand, PVP is a nontoxic, water-soluble, and synthetic biocompatible polymer. It is a suspending agent, protective colloid, clarifying agent, stabilizer, film former, viscosity modifier, and complexing agent. It is commonly introduced in many industrial applications as suspending agent, emulsion stabilizer, film former, and binder. PVP can be crosslinked through heating in presence of persulfates or at 150 °C [7, 8, 31, 32].
Keeping the above background in mind, the present work aims to emulsify castor oil using poly (N-vinyl-2- pyrrolidone)/ammonium persulfate system and apply the produced emulsion for functional finishing of cotton fabrics.
Experimental
Materials
Mill scoured and bleached cotton fabric of weave structure, weight of 128 g/m2 and count (Ne) of 40/1 was supplied by Misr Spinning and Weaving Co., Mahalla El—Kobra, Egypt. Castor oil (CAO) supplied by Iso-Chem. Company was used. Durapret LF (DMDHEU), low formaldehyde resin of conc. 70%, was used as a crosslinker for easy care finishing of cotton fabric. Poly (N-vinyl-2-pyrrolidone) (PVP) of molecular weights 40,000 and 1,300,000 Dalton was supplied by Alpha chemiKa. Silver nitrate, ammonium persulfate (APS) and ammonium sulfate (AS) of laboratory-grade chemicals were used.
Methods
Preparation of CAO /PVP/APS hybrid emulsion
The CAO/PVP hybrid emulsion was prepared as follows: 5 g of CAO and PVP, as 60% aqueous solution, in a specific weight ratio to CAO (0–20%) was mixed together in 150 ml beaker with stirring at different temperatures (60–90 °C). APS solution of specific concentration was then added to the reaction medium and the reaction was left to proceed under stirring for a period of time (0–90 min). After a certain time, specific volume of hot distilled water at ca 70 °C was added to the reaction medium followed by stirring using a strong homogenizer for 3 min to form a homogeneous CAO/PVP hybrid emulsion [2, 4].
Preparation of silver nano-particles (Ag-NPs)
Silver nano-particles (Ag-NPs) were prepared using trisodiumcitrate as a reductant as reported elsewhere [33].
Fabric treatment
Cotton fabric samples of 30X30 cm2 were padded twice in finishing bathes containing 60 g/L of DMDHEU, ammonium sulfate as a catalyst and different concentrations of CAO/PVP hybrid emulsion (0—40 g/L), to a wet pick up of 100%. The padded samples were dried at 100 °C/3 min in Wenner Mathis AGCH-8155 oven followed by curing at 150 °C/ 3 min. The finished fabrics were then washed with distilled water at 50 °C for 10 min, thoroughly rinsed and finally dried for testing.
Analysis and test methods
-
Fabric weight (W) was assessed according to ATSM (D 3776 – 79).
-
The wrinkle recovery angle of treated fabric samples, WRA, was determined according to ASTM method D-1296–98.
-
Tensile strength (TS) was tested in the warp direction according to ASTM procedure D-2256–98.
-
Wettability of the finished fabric sample was assessed according to AATCC Test method 39–1980.
-
The flexural rigidity test (FR) was carried out in the warp direction according to ASTM D 1388–64.
-
Surface roughness (SMD) was measured using Kawabata evaluation system, Surface tester KES-FB4-A, Kato Tech Co., LTD, Japan.
-
The antibacterial activity was assessed using the bacterial count method as reported elsewhere [12, 34, 35] against:
-
Gram-positive bacteria: Staphylococcus aureus (SA).
-
Gram-negative bacteria: Escherichia coli (EC).
-
Infra-Red (IR) spectroscopy was carried out using FT/IR-4700 FTIR Spectrometer from JASCO.
-
The morphology and particles size of the hybrid emulsion was obtained by transmission electron microscope (TEM) using a JEOL, JEM 2100 F electron microscope at 200 kV.
-
Scanning electron microscope (SEM) images of the treated and untreated fabric samples were obtained using SEM Model Quanta 250 FEG (Field Emission Gun) attached with EDX Unit (Energy Dispersive X-ray Analyses), with accelerating voltage 30 kV, magnification 14 × up to 1,000,000 and resolution for Gun, FEI company, Netherlands.
Results and discussion
Tentative mechanism
It was reported that heating of APS aqueous medium causes the decomposition of APS to free radical species (SO4−· and HO·) [1, 4, 7, 36, 37]. Thus, it is anticipated that introducing of PVP as well as castor oil in the previous reaction may cause the following reactions:


The aforementioned reaction contains a mixture of unreacted castor oil and PVP, castor oil grafted with PVP, crosslinked PVP in addition to other oxidized species; all in state of entanglement and will be donated by CAO/PVP/APS hybrid. Indeed, the hydroxyl groups as well as double bonds of castor oil act as grafting centers [38,39,40]. Moreover, the castor oil grafted with PVP may serve as a self emulsifier for the unreacted CAO whereas the crosslinked PVP act as a stabilizer for the resulted emulsion [40]. The major factors affecting that hybrid formation will be studied as follows.
Factors affecting the CAO/PVP/APS hybrid emulsion formation
APS concentration
Table 1 illustrates the impact of APS/CAO weight ratio on the CAO/PVP/APS hybrid emulsion state. It is obvious that, increasing APS/CAO weight ratio from 1 and up to 4% is accompanied with a gradual improvement in state of the hybrid emulsion which could be attributed to the gradual increasing in magnitude of the free radical species capable to form CAO/PVP species (Eq. 5) responsible for emulsification of the remaining CAO in the reaction medium [2,3,4]. Increase of the APS/CAO ratio in the reaction medium, within the range studied, does not affect the emulsion state, suggesting a rapid rate of termination due to the faster rate of initiation at the higher concentrations of APS [36, 37].
PVP/CAO weight ratio
Table 2 shows the effect of the PVP/CAO weight ratio, keeping the PVP concentration at 60%, on state of the CAO/PVP/APS hybrid emulsion. It is clear that increasing of the PVP/CAO ratio to 15% in the reaction medium results in a progressive improvement in the emulsion state which may be associated with the enhancement in CAO/PVP species formation capable to emulsify that hybrid [2,3,4]. The further increasing in such ratio, i.e. at 20%, does not change the hybrid emulsion state.
Reaction time
Table 3 reveals the state of the CAO/PVP/APS hybrid emulsion as a function of reaction time. It is clearly seen that increasing the reaction time up to 60 min brings about a gradual promotion in emulsion state of the hybrid, the matter that can be explained by the enhancement in APS decomposition and the subsequent increasing in CAO/PVP species formation that ultimately promotes the hybrid emulsion state. Beyond 60 and up to 90 min, the hybrid emulsion remains stable suggesting a depletion in both the PVP and/or APS concentrations [2,3,4, 36, 37].
Reaction temperature
The effect of the reaction temperature on emulsion state of the CAO/PVP/APS hybrid is shown in Table 4. It is well seen that raising the reaction temperature from 60 to 80 °C results in an upgrading the state of the hybrid emulsion, which can be associated with the enhancement in the initiator decomposition and the subsequent increasing in the free radicals number as well as increasing of mobility of the reactants molecules that subsequently enhances the CAO/PVP species formation responsible for emulsification of the unreacted CAO [2,3,4, 36, 37]. Moreover, increasing of the reaction temperature from 80 to 90 °C does not affect state of that hybrid emulsion suggesting a faster rate of termination at higher temperatures [36, 37].
PVP Molecular weight
The emulsion state of the CAO/PVP/APS hybrids prepared using PVP of molecular weights 40000 and 1300000 Dalton is shown in Table 5. It is clear that increasing of the PVP molecular weight over the range of 40000 to 1300000 Dalton results in formation of CAO/PVP/APS hybrids having stable emulsions. Moreover, upon storing both the hybrids emulsions for studying their emulsions stability, it was noticed that after about two weeks, tinny oil drops appeared on the CAO/PVP40000/APS hybrid emulsion surface which by shaking the emulsion, such oily drops disappeared and stable emulsion was obtained again. On the other hand, storing the CAO/PVP1300000/APS hybrid emulsion for one month does not change its visual appearance reflecting its higher stability compared to the CAO/PVP40000/APS hybrid emulsion.
Characterization of the of CAO/PVP/APS hybrids and their emulsions
IR of the CAO/PVP/APS hybrid
Figure 2 shows the FTIR analysis of CAO/PVP40000/APS hybrid, castor oil, and PVP. It is clear that the hybrid spectrum shows the following peaks:
-
1.
Peaks resembles to that of PVP spectrum that are [2, 4, 7, 8]:
-
A peak at 2923 cm−1 due to stretching vibration of -CH of PVP,
-
A peak at 1276 cm−1 due to C–N bond of PVP, and
-
A peak at 1666 cm−1 due to the stretching vibration the C = O group of both PVP and the crosslinked PVP.
-
-
2.
Peaks resembles to that of castor oil spectrum that are [1, 41,42,43,44]:
-
A broad stretching vibration band at 3397 cm−1 due to OH group,
-
A stretching vibration band at 3007 cm–1 due to C = C,
-
symmetric and asymmetric stretching vibration bands at 2923 and 2857 cm–1 respectively due to the of CH2,
-
A stretching band at 1739 cm–1 due to C = O,
-
Asymmetric and symmetric vibrations of –CH3 groups at 1450 and 1369 cm–1 respectively,
-
Stretching vibration bands at 1238 and 1160 cm–1 due to C–O of ester group, and
-
TEM images of the CAO/PVP/APS hybrids emulsions
Figure 3(A) and (B) shows the particles size of CAO/PVP40000/APS and CAO/PVP1300000/APS hybrids emulsions respectively determined by the transmission electron microscope (TEM). It is clear that the particles size of CAO/PVP40000/APS hybrid emulsion ranges approximately from 15 to 50 nm whereas that of CAO/PVP1300000/APS hybrid emulsion ranges from 270 to 350 nm suggesting that the higher the PVP molecular weight, the larger is the hybrid emulsion particle size.
Inclusion of the CAO/PVP/APS hybrids emulsion in easy care finishing of cotton fabric
Performance properties of treated fabric
Table 6 depicts the impact of inclusion of CAO/PVP40000/APS and CAO/PVP1300000/APS hybrids emulsions in easy care finishing bath of cotton fabric on some performance properties of treated fabric. It is well seen that crosslinking of cotton fabric samples in absence of that hybrids emulsions gives rise to an enhancement in resiliency accompanied with a reduction in tensile strength, softness degree, flexibility, i.e. higher rigidity, and wettability of treated fabric, compared with the untreated fabric. The matter that could be attributed to crosslinking of cotton cellulose with DMDHEU as a crosslinker and the subsequent molecular degradation in the cellulose structure [2,3,4,5].
Meanwhile, inclusion of either of the aforementioned hybrids emulsions in the above mentioned finishing bath brings about a fixation of such hybrids ingredients onto/within the treated fabric structure, via the labile hydrogen atom of the castor oil or its hybrid hydroxyl groups, [2,3,4,5,6] which subsequently results in promoting fabric softness, tensile strength and flexibility due the lubricating effect of the hybrid ingredients as well as reducing fabric wettabiliy and resiliency as a direct consequence for the decreasing in extent of crosslinking with the DMDHEU [6]. On the other hand and regardless of the hybrid type, increasing of the hybrid emulsion concentration in the aforementioned finishing bath leads to an enhancement in extents of the above mentioned properties suggesting the increasing in extent of fixation of such hybrids ingredients onto/within the treated fabric structure. Moreover, replacing the CAO/PVP40000/APS hybrid emulsion with the CAO/PVP1300000/APS hybrid emulsion in the finishing bath significantly enhances softness, tensile strength along with decreasing resiliency, wettability, and flexibility of treated fabric compared with the CAO/PVP40000/APS hybrid emulsion treated fabric which can be attributed to the difference between such hybrids in the PVP molecular weight [4].
Antibacterial activities of treated fabric
Table 7 shows the impact of the finishing bath formulation on the antibacterial properties of CAO/PVP40000/APS hybrid emulsion treated fabric. It is clear that incorporation of such hybrid emulsion gives rise to an enhancement in antibacterial bacterial properties of that hybrid emulsion treated fabric reflecting of the antibacterial properties of castor oil. Moreover, incorporation of Ag-NPs in the aforementioned finishing bath significantly improves extent of the antibacterial activities due to [32, 45, 46]:
-
(i)
Creation of Ag+, in a moisture presence, that binds to the bacterial DNA giving rise to its inactivation (Eq. 7), and/or
$${\mathrm O}_{2(\mathrm{aq})}+4{\mathrm H}_3\mathrm O^++{4\mathrm{Ag}}_{(s)}\rightarrow{{4\mathrm{Ag}}^+}_{(\mathrm{aq})}+6{\mathrm H}_2\mathrm O$$(7) -
(ii)
Genaration of oxygen radicals which subsequently oxidize the bacterial molecular structure (Eq. 8):
$${\mathrm{H}}_{2}\mathrm{O}+(1/2){\mathrm{O}}_{2}\stackrel{{\mathrm{Ag}}^{+}}{\to }{\mathrm{H}}_{2}{\mathrm{O}}_{2}\to {\mathrm{H}}_{2}\mathrm{O}+(\mathrm{O})$$(8)
Furthermore, Table 7 depicts that such antibacterial properties of treated fabric are durable for 15 washing cycles reflecting the crosslinker role in fixation of castor oil via its hydroxyl groups onto/within the finished fabric structure.
SEM and EDX images
Figure 4 shows the SEM images of untreated fabric and CAO/PVP40000/APS hybrid emulsion treated fabric. It is obvious the treated sample shows a deposited film of CAO/PVP40000/APS hybrid ingredients on its surface compared to the untreated sample image. On the other hand, Fig. 5 loading the treated fabric with Ag-NPs of a content 0.09% (w/w).
Conclusions
-
The optimum reaction conditions to synthesize the CAO/PVP/APS hybrids are: PVP/CAO weight ratio, 15%; PVP concentration, 60%; APS conc., 4% (based on weight of CAO); reaction temperature, 80 °C; reaction time, 60 min.
-
The FTIR analysis confirmed synthesis of the CAO/PVP/APS hybrids.
-
The TEM analysis proved that the particles of the CAO/PVP1300000/APS hybrid emulsion are larger than that of the CAO/PVP1300000/APS hybrid emulsion.
-
Incorporation of either of the aforementioned hybrids emulsions in the easy care finishing formulations of cotton fabric results in promoting fabric softness, tensile strength, flexibility, and antibacterial activities of treated fabric along with reducing fabric wettabiliy and resiliency.
-
Inclusion of Ag-NPs in the aforementioned finishing bath significantly improves extent of the antibacterial activities of treated fabric.
-
The treated fabric was examined using SEM as well as EDX analysis.
References
Fahmy HM, Amr A, Aly AA, Sayed SM (2019) Synthesis of castor oil/2,4-toluene diisocyanate adduct to impart water repellency and antibacterial properties for cotton/polyester fabric. J Coat Technol Res 16(1):31–39
Fahmy HM, Aly AA, Sayed ShM (2017) Graft copolymerization of N-vinylpyrrolidone onto stearyl alcohol to impart water repellency and antibacterial properties for cotton/polyester fabric. Prog Org Coat 105:176–182
Fahmy HM, Aly AA, Amr A, Sayed ShM, Rabie AM (2016) SA/TDI/PEG adducts as water repellent finishes for cotton/polyester blended fabric. Prog Org Coat 99:166–172
Fahmy HM, Aly AA, Amr A, Sayed ShM, Rabie AM (2016) An innovative SA/PVP hybrid for attaining water repellent cotton/polyester blended fabric. Int J ChemTech Res 9(3):215–227
Abo-Shosha MH, El-Sayed Z, Fahmy HM, Ibrahim NA (2005) Synthesis of PEG/TDI/F6 adducts and utilization as water/oil repellents and oily stain release finishes for cotton fabric. Polym Plastic Technol Eng 44:1189
Abo-Shosha MH, Ibrahim NA, Fahmy MH, Hebeish A (1995) Utilizing water soluble size additives in easy care finishing. Am Dyest Rep 84(7):44
Fahmy HM (2009) Utilization of Poly (N-vinyl-2- pyrrolidone) in easy care finishing of cotton fabrics to improve their performance properties and antibacterial activities. J Ind Text 39(2):109
Fahmy HM, Abo-Shosha MH, Ibrahim NA (2009) Crosslinking of cotton fabrics thermally with PVP to improve their performance and antibacterial properties. Carbohyd Polym 77:845
Ibrahim NA, Fahmy HM, Rehim MA, Sharaf SS, Abo-Shosha MH (2010) Finishing of Cotton Fabrics with Hyperbranched poly (ester-amine) to enhance their antibacterial properties and UV protection. Polym Plast Technol Eng 49:1297–1304
Ibrahim NA, Aly AA, Eid BM, Fahmy HM (2018) Green approach for multifunctionalization of cellulose-containing fabrics. Fibers Polym 19(11):2298–2306
Abdel-Wahab BF, Gaffer HE, Fouda MMG, Osman EM, Fahmy HM (2009) Synthesis of some new 2-(2,3-dihydroinden-1-ylidene) hydrazinyl)-4-methylthiazole derivatives for simultaneous dyeing and finishing for UV protective cotton fabrics. J Appl Polym Sci 112:2221
Ibrahim NA, Amr A, Eid BM, Mohamed ZE, Fahmy HM (2012) Poly(acrylic acid)/poly(ethylene glycol) adduct for attaining multifunctional cellulosic fabrics. Carbohyd Polym 89:648–660
Fahmy HM, Eid RAA, Hashem SS, Amr A (2013) Enhancing some functional properties of viscose fabric. Carbohyd Polym 92:1539–1545
Ibrahim NA, El-Sayed Z, Fahmy HM, Hasabo AGA, Abo-Shosha MH (2013) Perfume finishing of cotton/polyester fabric crosslinked with DMDHEU in presence of softeners. RJTA 17(4):58–63
Ibrahim NA, Abo-Shosha MH, Fahmy HM, El-sayed ZM, Hebeish AA (2008) Hybrids for finishing cotton fabric with durable handle performance. J Mater Process Technol 200:385
Fahmy HM, Okda HMY, Hassabo AG, El-Rafie MH, Youssef MA (2021) Synthesis of new silicone-based adducts to functionalize cotton fabric. Silicone
Gupta D (2013) Softening treatments for technical textiles, In: Advances in the dyeing and finishing of technical textiles, Edited by M. L. Gulrajani, Woodhead Publishing
Choudhury AKR (2017) Principles of textile finishing. Woodhead Publishing, The Textile Institute Book Series
Choudhury AKR, Chatterjee B, Saha S, Shaw K (2012) Comparison of performances of macro, micro and nano silicone softeners. J Text Inst 103:1012–1023
Ogunniyi DS, Fakayejo WRO, Ola A (1998) Industrial utilization of castor oil: polyurethane foam synthesis and properties. J Niger Soc Chem Eng 17:40–43
Jena J, Gupta AK (2012) Ricinus communis Linn: a phytopharmacological review. Int J Pharm Pharm Sci 4(4):25–29
Patel VR, Dumancas GG, Viswanath LCK, Maples R, Subong BJJ (2016) Castor oil: properties, uses, and optimization of processing parameters in commercial production. Lipid Insights 9:1–12
Gayki BK, Thorat PV, Tayde SS (2015) A review on synthesis and characterization of castor oil based polyurethane adhesive. Int J Emerg Technol Adv Eng 5(3):95–97
Nielsen FS, Petersen KB, Müllertz A (2008) Bioavailability of probucol from lipid and surfactant based formulations in minipigs: influence of droplet size and dietary state. Eur J Pharm Biopharm 69(2):553–562
Ma F, Bell AE, Davis FJ, Chai Y (2015) Effects of high hydrostatic pressure and chemical reduction on the emulsification properties of gum Arabic. Food Chem 173:569–576
Golkar A, Nasirpour A, Keramat J, Desobry S (2015) Emulsifying properties of angum gum (Amygdalus scoparia Spach) conjugated to β- lactoglobulin through maillard-type reaction. Int J Food Prop 18(9):2042–2055
Zhang D, Lin Y, Li A, Tarasov VV (2011) Emulsification for castor biomass oil. Front Chem Sci Eng 5(1):96–101
Tomasino C (1992) Chemistry and technology of fabric preparation and finishing. North Carolina State University, North Carolina, USA, College of Textiles
Nair G, Jadeja Y, Donga M, Vaghasiya D, Vora V (2019) Production of eco friendly fabric softener. Int J Appl Eng Res 14(1):8–15
Fahmy HM, Almetwally AA (2023) Emulsification of castor oil using poly (N-vinyl-2-pyrrolidone) for functional finishing of cotton fabric. J Eng Fibers Fabr 18:1–13
Blecher L, Lorenz DH, Lowd HL, Wood AS, Wyman DP (1980) Polyvinylpyrrolidone. In: Davidson RL (ed) Handbook of Water-Soluble Gums and Resins. McGraw-Hill Book Company, New York, pp 21–22
Can HK, Kirci BU, Kavlak S, Uner AG (2003) Removal of some textile dyes from aqueous solutions by poly (N-vinyl-2-pyrrolidone) and poly (N-vinyl-2-pyrrolidone)/K2S2O8 hydrogels. Radiat Phys Chem 68:811–818
Sileikaite A, Prosycevas I, Puiso J, Juraitis A, Guobiene A (2006) Analysis of silver nanoparticles produced by chemical reduction of silver salt solution. Mater Sci 12:287–289
Fahmy HM, Aly AA, Sayed ShM, Abou-Okeil A (2021) К-carrageenan/Na-alginate wound dressing with sustainable drug delivery properties. Polym Adv Technol 32(4):1793–1801
Abou-Okeil A, Fahmy HM, Fouda MMG, Aly AA, Ibrahim HM (2021) Hyaluronic acid/oxidized к-carrageenan electrospun nanofibers synthesis and antibacterial properties. BioNanoScience 11(3):687–695
Ibrahim NA, Hebeish A, Fahmy HM, Abo-Shosha MH (2006) Synthesis characterization and application of poly (acrylamide) / poly(vinyl alcohol) polyblends. Polym Plastic Technol Eng 45:341
Hebeish A, Fahmy HM, Abo-Shosha MH, Ibrahim NA (2006) Preparation of new chemical polyblend sizing agent via polymerization of acrylic acid with polyvinyl alcohol. Polym Plastic Technol Eng 45:309
Ouellette RJ, Rawn JD (2015) 15-Synthetic Polymers. In: Ouellette RJ, Rawn JD (eds) Principles of Organic Chemistry. Elsevier, Boston, pp 397–419
Kunduru KR, Basu A, Zada MH, Domb AJ (2015) Castor oil-based biodegradable polyesters. Biomacromol 16:2572–2587
Haaf F, Sanner A, Straub F (1985) Polymers of n-Vinylpyrrolidone: synthesis, characterization and uses. Polym J 17:143–152
Dias JM, Araújo JM, Costa JF, Alvim-Ferraz MCM, Almeida MF (2013) Biodiesel production from raw castor oil. Energy 53:58–66
Boey P-L, Saleh MI, Sapawe N, Ganesan S, Maniam GP, Ali DMH (2011) Pyrolysis of residual palm oil in spent bleaching clay by modified tubular furnace and analysis of the products by GC–MS. J Anal Appl Pyrolysis 91:199–204
Mirghani MES, Man YBC (2003) Determination of hexane residues in vegetable oils with FTIR spectroscopy. J Amer Oil Chem Soc 80:619–623
de la Mata P, Dominguez-Vidal A, Bosque-Sendra JM, Ruiz-Medina A, Cuadros-Rodríguez L, Ayora-Cañada MJ (2012) Olive oil assessment in edible oil blends by means of ATR-FTIR and chemometrics. Food Control 23:449–55
Fahmy HM, Ali AA, Abou-Okeil A (2021) Simple Way for Ag-NPs Preparation based on starch macromolecule. Lett Org Chem 18(12):1–8
Fahmy HM, Aly AA, Abou-Okeil A (2018) A non-woven fabric wound dressing containing layer – by – layer deposited hyaluronic acid and chitosan. Int j Biol Macromol 114:929–934
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicting interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fahmy, H.M., Amr, A. Synthesis of castor oil/poly (N-vinyl-2-pyrrolidone)/ammonium persulfate hybrids emulsion as textile softeners. J Polym Res 30, 400 (2023). https://doi.org/10.1007/s10965-023-03779-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03779-3