Abstract
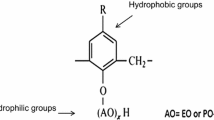
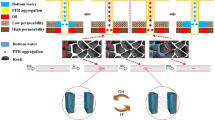
The stability of phenol–formaldehyde resin (nHAP) in the oil displacement process influences its flooding and profile control capabilities. There aren't many studies that compare how various surfactants affect PFR stability. The surfactants CTAB, Tween 60, and SDS were selected as representative ones. To look at the changes in stability, spectral turbidity and dynamic light scattering experiments were employed. It was found that the cationic surfactant CTAB can connect with the surface of PFR, just as metal cations, lowering electrostatic repulsion and facilitating the aggregation of PFR composite molecules. Anionic and nonionic surfactants both have some degree of system stability stabilizing properties. When Tween 60 and SDS adsorbed on the surface of the PFR molecule formed a hydrogen bond network with the hydroxymethyl or phenolic hydroxyl group, for example by hydrogen bond interaction, the PFR molecule's dispersion stability was increased. The Tween 60 and PFR composite systems have bigger particle sizes in addition to being more stable. Additionally, the energy barrier of the Tween 60/PFR composite system is determined using the modified DLVO theory for Lewis acid–base hydration, and it is discovered to be consistent with the experimental findings. The findings demonstrate that the stability of the composite system can be impacted by changes in the hydrophilicity and hydrophobicity of the composite system. The findings demonstrate that the stability of the composite system can be impacted by changes in the hydrophilicity and hydrophobicity of the composite system. Understanding the co-migration of PFR and surface activity during oil displacement depends heavily on the data.












Similar content being viewed by others
Abbreviations
- PER:
-
Phenol-formaldehyde resin
- SDS:
-
Sodium dodecyl sulfate
- CTAB:
-
Cetyltrimethyl ammonium bromide
References
Kumar P, Choonara YE, Pillay V (2018) In silico analytico-mathematical interpretation of biopolymeric assemblies: Quantification of energy surfaces and molecular attributes via atomistic simulations. Bioeng Transl Med 3(3):222–231
Han X, Kurnia I, Chen Z, Yu JJ, Zhang GY (2019) Effect of oil reactivity on salinity profile design during alkaline-surfactant-polymer flooding. Fuel 254
Vu T, Reynolds G, Hutton HD, Kasting GB, Koenig P (2021) Rheology control using nonionic cosurfactants and pH titration in an amino acid-derived surfactant composition. Langmuir 37(42):12327–12334
Gumaste SG, Gupta SS, Serajuddin AM (2016) Investigation of polymer-surfactant and polymer-drug-surfactant miscibility for solid dispersion. AAPS J 18(5):1131–1143
Benhur AM, Pingali S, Amin S (2020) Application of biosurfactants and biopolymers in sustainable cosmetic formulation design. J Cosmet Sci 71(6):455–480
Abdullahi MB, Jufar SR, Kumar S, Al-shami TM, Negash BM (2022) Synergistic effect of Polymer-Augmented low salinity flooding for oil recovery efficiency in Illite-Sand porous media. J Mol Liq 358
Zhao D, Li MY, Peng B, Lin MQ, Dong ZX (2013) Study on the effect of Tween 60 on dispersion property of water-soluble phenol-formaldehyde resin. J Dispers Sci Technol 34(8):1085–1091
Nagy R, Bartha L, Toth J, Dudas J, Vago A (2017) Investigation of the interaction between polymer and surfactant aqueous solution for the petroleum industry. Pet Sci Technol 35(4):360–364
Muneer R, Hashmet MR, Pourafshary P (2022) Predicting the critical salt concentrations of monovalent and divalent brines to initiate fines migration using DLVO modeling. J Mol Liq 352
Eskhan AO, Abu-Lail NI (2020) Force-averaging DLVO model predictions of the adhesion strengths quantified for pathogenic listeria monocytogenes EGDe grown under variable pH stresses. Langmuir 36(30):8947–8964
OrtegaVinuesa JL, MartinRodriguez A, Hidalgo-Alvarez RH (1996) Colloidal stability of polymer colloids with different interfacial properties: Mechanisms. J Colloid Interface Sci 184(1):259–267
Li Q, Wang Q, Hou J, Zhang J, Zhang Y (2021) Aggregating structure in coal water slurry studied by eDLVO theory and fractal dimension. Front Energy. https://doi.org/10.1007/s11708-021-0736-1
Iamazaki ET, Schmitt CC, Neumann MG (2001) Photophysical study of the interactions of charged copolymers with surfactants of opposite charge. Langmuir 17(11):3486–3490
Philip J, Gnanaprakash G, Jayakumar T, Kalyanasundaram P, Raj B (2003) Three distinct scenarios under polymer, surfactant, and colloidal interaction. Macromolecules 36(24):9230–9236
Mahajan RK, Vohra KK, Aswal VK (2013) Structural behavior of aggregate assemblies of cationic surfactants and their mixtures with triblock polymers. J Dispers Sci Technol 34(2):244–251
Paternina CA, Londono AK, Rondon M, Mercado R, Munoz S (2019) Influence of salinity and hardness on the micellization of an extended surfactant studied by turbidimetry. J Pet Sci Eng 182
Zhao Y (2009) Spacer-dependent folding and aggregation of oligocholates in SDS micelles. J Org Chem 74(19):7470–7480
Tyagi G, Greenfield JL, Jones BE, Sharratt WN, Khan K, Seddon D, Malone LA, Cowieson N, Evans RC, Fuchter MJ, Cabral JT (2022) Light responsiveness and assembly of arylazopyrazole-based surfactants in neat and mixed CTAB micelles. JACS Au 2(12):2670–2677
Weng T, Wang L, Liu Y, Zhang X, Wu Y, Zhang Y, Han J, Liu M (2022) Interaction of bisdemethoxycurcumin with sodium dodecyl sarcosine + Tween 20/Tween 60 mixed surfactants: Insights from multispectral analysis and solubilization effect. Colloids Surf A 645:128928
Yu Y, Ma L, Xu H, Sun X, Zhang Z, Ye G (2018) DLVO theoretical analyses between montmorillonite and fine coal under different pH and divalent cations. Powder Technol 330:147–151
Zhao D, Yang H, Li Z, Yang W, Li G, Wei Y, Zhang S, Tang Z, Wang L, Li J, Feng H (2022) Effects of the same valence metal cations on the aggregation behavior of PFR. React Funct Polym 179:105363
Zhao D, Li M, Peng B, Lin M, Dong Z (2013) Study on the effect of Tween 60 on dispersion property of water-soluble phenol-formaldehyde resin. J Dispers Sci Technol 34(8):1085–1091
Acknowledgements
The Natural Science Foundation of China (Grant numbers: 2020IM030400), the Special Project on Innovative Methods Fund Program of the Ministry of Science and Technology of the People's Republic of China, and the Natural Science Foundation of China all provided financial support for this work (Grant numbers: 21664009,51063003). We appreciate Dr. Huixia Feng's insightful conversation. We are grateful that the PetroChina Lanzhou Lubricating Oil R&D Institute provided the measurement tools.
Funding
The financial support from the Natural Science Foundation of China (Grant numbers: 21664009,51063003), the Natural Science Foundation of China (Grant numbers: 2020IM030400) and the Ministry of Science and Technology of the People's Republic of China, Special Project on Innovative Methods Fund Program (No. 2020IM030400) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Dan Zhao, Haoling Yang and Yuanyuan Wei performed the measurements, Dan Zhao and Huixia Feng were involved in planning and supervised the work, Dan Zhao and Haoling Yang processed the experimental data, performed the analysis, drafted the manuscript and designed the figures. Zhongping Tang and Liping Wang performed the fractal dimension calculations. Weili Yang and Zhaoyang Li manufactured the samples and characterized them with high performance liquid chromatography-mass spectroscopy, Wenzhe Yang and Jin Li performed the infrared spectrum. Huixia Feng aided in interpreting the results and worked on the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10965_2023_3495_MOESM2_ESM.docx
Supporting Information includes calculation information for the fractal dimension Infrared Spectrum and DLVO as well as High Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) (DOCX 882 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, D., Yang, H., Wei, Y. et al. Molecular modeling of phenol formaldehyde resin—surfactant and its dispersion stability in salt solution. J Polym Res 30, 131 (2023). https://doi.org/10.1007/s10965-023-03495-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03495-y