Abstract
The function of a membrane in the fuel cell is critical to its success. The major component of a direct methanol fuel cell (DMFC) is the proton exchange membrane (PEM) which must have proton conductivity, thermal stability, mechanical qualities, and low methanol permeability. In this study case, the film-forming and structural properties of Polyvinyl chloride (PVC) impelled us to employ them for developing polyelectrolyte membranes (PEMs). To functionalize the resultant PEMs, Graphene oxide (GO) and zirconium phosphate (ZrP) were incorporated into polyvinyl chloride in different proportions. The structural and physical properties of PVC/GO-ZrP membranes were investigated by using a variety of techniques instance, Fourier transform infrared spectroscopy (FTIR), Scanning electron microscope (SEM), Transmission electron microscope (TEM), Thermogravimetric analyzer (TGA), universal testing machine, and water contact angle meter. Furthermore, water uptake, Methanol uptake, and ion exchange capacity (IEC) were measured. The results demonstrated that the membranes developed have enough characteristics to be valid in DMFCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population growth has soared worldwide energy consumption by leaps and bounds in recent years. As a result, conventional energy supplies, particularly nonrenewable ones, are in short supply. As a result, there is a global push to use sustainable and eco-friendly energy sources [1]. The membrane-based fuel cell is a revolutionary sustainable energy source with high efficiency, minimal resource use, a compact structure, low noise, and no pollutant emissions.
DMFCs are now of the utmost interest of these fuel cells, mainly for applications of portable batteries, because of their low-temperature and high-power density device and eco-friendly, remarkable efficiency, and cheap fuel use in transportation [2].
Furthermore, PEM is a critical component of DMFC systems since it works as a barrier to fuel crosslinks across electrolytes and for moving protons from anode to cathode.
Ionomeric polymers (ionomers) and PEMs have been employed in batteries, sensors, and low-temperature cells, among other electrochemical devices. Alternative energy is one conceivable application for (PEMFCs).
Lastly, there has been much interest in developing (PEMFC), mainly for vehicle power applications. In a PEMFC, a proton electrolyte membrane is placed between two porous electrodes with a catalyst [3]. The PEMFC is critical to the advancement of fuel cell technology. It has good proton conductivity as well as long-term mechanical stability. Nafion® N-117, a per-fluorinated ionomer-based polymer with high chemical, thermal, and physical stability as well as high proton conductivity, is one commercially available PEM that has proven to be useful in PEMFC applications [4]. Aside from these benefits, the Nafion® membrane has two drawbacks:
They are quite expensive due to the risky and difficult manufacturing process, and they demonstrated greater methanol permeability in (DMFCs) [5]. High methanol cross-over reduces both fuel usage efficiency and cathode performance. These drawbacks have sparked interest in alternative hydrocarbon polymer-based membranes [6].
The polymer electrolyte membranes were made of (PVC) polymer. In contrast, composite membranes were fabricated from inorganic materials, including silica, titania, zirconia, phosphotungstic acid (PWA), graphene oxide (GO), silicotungstic acid (SWA), montmorillonite (MMT), kaolinite, and hydroxyapatite (HAP) [7]. (PVC) was chosen due to its excellent chemical and physical features. In terms of properties of physical and chemicals, ion-exchange organic–inorganic membranes with an organic substrate and a dispersed inorganic phase containing functional (ionic) groups outperform both organic and total membranes inorganic, and they are likely to be employed in DMFC [8]. The phase inorganic is chosen according to its (IEC) and conductivity [9]. Fast proton conductors are a type of inorganic ion exchanger that has recently gained popularity.
These compounds are acid salts of tetravalent metals with structure [M (IV) HPO4 nH2O], where M, such as Zr, Sn, or Ti, is a tetravalent metal. The metal is chemically connected to the hydroxyl group's protons (H+) (O.H.-). M serves as ion-exchangeable sites, permitting proton conduction. A high IEC can be achieved by exchanging several minor cations (Li+, Na+, Ca2+, and so on) with (H+). Protons freely travel on surfaces with hydrated (O.H.-) groups [10, 11]. Zirconium phosphate (ZrP) is a fast proton conductor due to its IEC is high and thermal durability up to 450 °C. However, its proton conductivity is relatively low [12]. Polytetrafluoroethylene (PTFE), polyvinyl chloride (PVC), polyethersulfone (PES), and polypropylene (PP) as polymeric materials are impregnated with a colloidal suspension of inorganic material to produce inorganic–organic composite membranes [13]. According to Previous research, particle size is small (varying from nano to micrometers), and the transfer of ZrP dispersion colloidal particles into a matrix allows inorganic particle dispersion [14] [15]. Solid inorganic proton conductors that are hydrophilic and proton conductors include Heteropoly acids (HPAs), metal hydrogen sulfates, and zirconium phosphates. Low prices, great mechanical in dry and wet states, and good thermal stability are all advantages of composite membranes [16, 17]. We improved the chemical and physical properties of the membranes by using graphene oxide and zirconium Phosphate (ZrP) (by converting Zirconium oxychloride on the membrane surface), as well as the method of introducing proton conductors into the polymer, and the membrane was prepared by solvent evaporation.
The thermal and chemical stabilities of prepared (PVC-GO/ZrP) membranes were excellent. It demonstrated outstanding thermal stability up to a temperature of 250 °C, which is sufficient for fuel cell operations because the fuel cell worked between 80 and 140 °C. All the foregoing results show that the PVC-GO/ZrP membrane is suitable for electrochemical applications [18].
Materials and methods
Materials
Fine powder of Polyvinyl chloride (M.W. ~ 48,000) from Belami fine chemicals (Mumbai, India). Graphite. Tetrahydrofuran (THF) (purity 99.9%) from Panreac (E.U.). Sulfuric acid (purity 98%, Mw = 98.08 g/mole) obtained from United company. Ortho-Phosphoric acid (Mw = 98 g/mole; purity 85%; density 1.721 g/ml) from Poland. Potassium Permanganate (KMnO4), (Mw ~ 158.03; Assay = Min. 99.0%), Spectrum chemicals MFG.CORP. Zirconium (IV) oxychloride octahydrate (ZrOCl2.8H2O, MW ~ 322.25; Assay = 99.5%) from Euromedex- France. Sodium chloride (purity 99.9%) was purchased from Raheja center, India. Sodium Hydroxide (pellets, purity 98%) was obtained from Central Drug House Ltd (New Delhi, India). Chloroform (Mw ~ 119.38; Assay 99%) from Fisher Chemical, Germany. Absolute ethanol (99.8%) from Sigma-Aldrich, Germany. Methanol (99.5%) from Sigma-Aldrich, Germany. All other compounds were utilized as received without being purified further.
Fabrication of (GO) from natural purified Graphite
The GO flakes were created using purified natural Graphite's modified Hummers' technique [19]. The first 3.0 g of flakes of Graphite was mixed with sodium nitrate (1.5 g) and sulfuric acid concentrated (69 ml). The mixture froze in an ice bath to 0 °C then, potassium permanganate (9.0 g) was slowly added. After that, the temperature was raised to 35 °C and 2 h of stirring. Afterward, de-ionized (DI) water (138 ml) was gently added, and then, retain the reaction conditions by heating for fifteen minutes at 98 °C. The solution was chilled for 10 min in a water bath. Then, treatment with 420 mL of (H2O) and 3 mL of 30% hydrogen peroxide (H2O2) occurred in the solution. After cooling the solution, the supernatant acidic was centrifuged off, and washed the leftover mixture with ethanol and hydrochloric acid until a neutral pH was reached after washing with D.I water. The leftover material was diluted softly and sonicated [20]. (Scheme 1) demonstrates the preparation of graphene oxide from natural Graphite.
Preparation of Graphene Oxide from Natural Graphite [21]
Preparation of composite membrane from (PVC/GO)
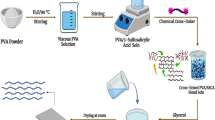
PVC/GO composite membrane was prepared using the colloidal mixing procedure. Figure 1 depicts a protocol synthesis in which PVC was dissolved with a definite amount at 70 °C in THF, and GO powder Separately was dispersed and sonicated in THF for 1 h. Then, sonicated the mixture for another hour. The resulting dispersion homogeneous solution was cast in a petri dish glass and dried at room temperature, yielding flawless PVC/GO composite films [22]. (Scheme 2) demonstrates the bonding interaction between PVC and GO in schematic form, illustrating that chemical bonding occurs as a result of an ester linkage formation. Previous studies have demonstrated that halogen bonding can be obtained via the GO (which has an oxygen-containing group) and PVC [23, 24] and can operate as an active site to anchor functional groups, thereby rendering compatibility between GO and PVC [25].
Bonding interaction between GO and PVC [22]
Preparation of ZrP ion-exchanger
By stirring 20 ml of H3PO4 solution (0.5 M) with 10 (wt.%) of Zirconium oxychloride for 5 min, ZrP powder was synthesized. To keep the pH between 2 and 3, HCl was added to the solution with a few drops. Then, stirred the solution at room temperature for 2 h, and the solution became milky. After that, the precipitate was filtered and washed many times with (DI) water to produce the ZrP powder by removing excess acid before being roasted at 120 °C for 12 h.
Preparation of (PVC/GO-ZrP) membrane
The PVC/GO-ZrP membrane was prepared in two steps; first, the prepared PVC/GO membrane was immersed in various concentrations of ZrOCl2 aqueous solution (5, 7.5, 10, 12.5, 15 wt. %) separately for 6 h at 80 °C. Second, after removing excessive solution from the surface, the ZrOCl2 permeated PVC/GO membrane was soaked in H3PO4 (0.5 M) for 48 h. followed by heating at 120 °C for 12 h. for ZrP creation on the PVC/GO membranes surface. [26].
Membranes characterization properties
Ion Exchange Capacity (IEC)
The IEC shows the proportion of hydrophilic ionizable groups in the membrane matrix, which are responsible for the membranes' ionic conductivity. The membranes' (IEC) were evaluated using acid–base titration. Membrane specimens with a definite weight were immersed for 24 h in NaCl (2 M).
Titration with phenolphthalein and sodium hydroxide (0.01 N) was used to determine the protons emitted from these samples. The following equation was used to calculate IEC: \(\mathbf{I}\mathbf{E}\mathbf{C}(\mathbf{m}\mathbf{e}\mathbf{q}/\mathbf{g})=\frac{\mathbf{N}\times \mathbf{V}}{\mathbf{W}}\)
Where N, W, and V denote the concentration of the NaOH solution, the sample weight, and the titer of the NaOH, respectively [27].
Water uptake and methanol uptake
The sample was put for 24 h in de-ionized water at room temperature before being withdrawn from the water. The filter paper was used to remove the water from the surface by wiping the sample with it; then, the samples were weighed immediately. The following equation determined the water uptake:
where Wdry and Wwet were the weights of the dry and wet membranes, respectively, a standard approach for testing methanol uptake involved substituting water with methanol. [28].
Tensile strength measurement
A universal testing machine (model AG-I, Shimadzu, Kyoto, Japan) was used to measure the tensile strength (TS) and elongation at break (E) of the polymer-based membranes at room temperature. The measurements were conducted with the cross-heads moving at a fixed rate (3 mm/min.) [29].
Water contact angle measurement
VCA contact angle device 2500 XE with analytic software (AST Products, Billerica, MA) and a CCD camera investigated the hydrophilicity of polymer blend-based membranes [30].
FT-IR spectral investigation
FT-IR Spectral analysis was used to investigate the structure of the polymeric substances and to confirm the success of the synthesis process in the range of 400–4000 cm−1, with a scanning rate of the 30 s and resolution of 4 cm−1 using (Shimadzu FT-IR-8400 S, Japan) [31].
X-ray Diffractometry (XRD)
Shimadzu (XRD-6000) device with a back monochromatic and a Cu Anticathode. The scan radical was performed in reflection scanning mode with 2θ moved from 0 to 120° [32].
Scanning Electron Microscopy (SEM)
The microstructure and morphological characteristics of polymer-based membranes were examined using JEOL JSM-6360LA, SEM, Japan. Samples of the resulting membranes were tested at a 10 K from 500 to 5,000 magnification power [33].
Transmission Electron Microscopy (TEM)
(TEM) Graphite, graphene oxide, and ZrP were performed using a JEM-2000EX (Japan) at a 200 kV acceleration voltage. Before measurement, the suspended particles in ethanol were transferred to a copper grid (400 meshes) coated with a carbon film [34].
Thermogravimetric analysis (TGA)
Thermal degradation characteristics of polymer blend-based membranes were determined by the TGA device (Shimadzu TGA-50 at a rate of 10 °C/min and 800 °C ambient temperature [35].
Results & discussion
Ion Exchange Capacity (IEC)
Ion exchange capacity indicates the number of exchangeable protons in the polymer matrix, which determine the proton conductibility. Therefore, the aforementioned prepared membranes were measured at room temperature. Pristine PVC membrane has no ion exchange capacity because it has no ionized functional groups in its backbones.
The IEC values of prepared PVC/GO composite membranes were measured and recorded in Table 1. It was observed that the IEC values were increased with increasing the proportion of GO, which possesses carboxyl groups that are responsible for IEC.
The IEC values of PVC/GO-ZrP membranes were determined and presented in Table 2. As expected, the IEC values were increased patently from 0.27 to 0.75 meq/g due to the modification (PVC/GO) membrane by immersing it in different concentrations in ZrOCl2 solutions ranging (from 5—15 wt.%), and this resulted in the formation of ZrP on surface, and thus increased IEC values due to the presence of phosphoric acid groups through the formed zirconium phosphate.
It was observed that the highest IEC value of 0.75 meq/g was recorded for PVC/GO-ZrP membrane at (15% ZrOCl2) due to the increase in the degree of phosphorylation of PVC/GO and/or increase in the proportion of ZrP in the blends. The electrochemical approach for determining the membrane's surface charge and the total number of exchangeable ion sites in the membrane is measured by IEC [36, 37] (Figs. 2, 3, 4, 5 and 6).
Water uptake and methanol uptake
Polyelectrolyte membrane (PEM) proton conductivity is greatly influenced by its water amount, and hydration is a critical step toward improving the fuel cell's efficacy by enhancing its conductivity [38]. To eliminate fuel cross-over in DMFCs that use methanol as a source of hydrogen, the polyelectrolyte membrane's methanol absorption must be lowered [28].
Therefore, water and methanol uptake of prepared PVC/GO composite membranes were determined and presented in Table 3. It was observed that the percentage of water uptake of PVC/GO membranes was enhanced by increasing the molar concentration of GO., which possess hydroxyl groups, which are hydrophilic groups responsible for hydrophilicity and increasing water uptake. It can be shown that the water uptake values of PVC (2%) membranes at various GO concentrations exhibit the highest values when compared to other PVC membrane concentrations. Likewise, the values of water uptakes of PVC/GO-ZrP membranes were increased by increasing the ZrP concentrations, as shown in Table 4. It is observed that the water uptake percentage increases in the presence of ZrP because this leads to the presence of phosphoric acid groups on the surface of the membrane in addition to the presence of hydroxyl groups and carboxylic acid groups from GO, which improves the hydrophilicity of membranes and thus increase water absorption [39]. The highest water uptake was 25.91% for PVC (3%) /GO (1.5%)-ZrP at (15% ZrOCl2) membrane and this is a lower value in comparison with the water uptake for Nafion-117 (29.6%) [39].
In general, water uptake of the resultant membranes is significantly linked to the presence of hydrophilic (carboxylic and phosphoric) groups in the polymer matrix, as evidenced by IEC and contact angle measurements. On the other hand, pre-prepared membranes with a larger water-holding capacity had a lower methanol uptake. These observations can relate to polymer matrices and solvents' hydrophilic/hydrophobic behavior. [40] (Table 5).
Mechanical properties
Mechanical characteristics are regarded as one of the most important elements in determining the potential efficiency of DMFCs. Rectangular strips' elongation and tensile strength at break were estimated in the dry state. Tensile strength denotes the highest stress created in a membrane during a tensile test, whereas elongation denotes the membrane's ability to stretch.
Tensile elongation indicates the ability of a material to deform before breaking; it is a more important design factor in choosing a proper material than many of the average properties…" [41]. Later Menges and Boden [42] also reported on the significance of the relationship between elongation and brittleness. The brittleness of materials, whether naturally or aging, significantly affects performance and manifests itself in various properties. We can see from this that the elongation at break is important to brittleness. A material with high elongation at break will not be brittle; thus, an inverse relationship is proposed [43]. The mechanical characteristics of Pristine PVC and PVC/GO composite membranes were investigated, and the results are displayed in Table 6. The influence of tensile strength and membrane elongation was detected as good results as the elongation of the membrane increased due to the incorporation of GO into the PVC membrane, signifying that the PVC/GO composite membrane became more elastic. The mechanical characteristics of pure PVC membranes have been enhanced in general.
The tensile properties of prepared PVC/GO-ZrP composite membranes were measured and presented in Table 7. These results indicated that increasing the ZrP content improves the mechanical properties of the PVC/GO films; These results are attributed to the addition of ZrP to PVC/GO films, which may enhance the mechanical properties to a certain extent; However, continuous addition of ZrP to PVC/GO films resulted in mechanical decline of the films.
It was observed that the PVC/GO-ZrP membrane at (12.5% ZrOCl2) has a tensile strength of 20.91 ± 0.6 (MPa), which is higher than that of the Nafion-117 membrane, which has a tensile strength of 18.125 (MPa) [44].
Finally, we have found that adding GOMand ZrP particles to a polymer matrix may change the brittleness of the pure polymer. The change, whether an increase or decrease, depends on multiple factors (particle dispersion, filler matrix adhesion, the amount of filler added, etc.), and typically (but not always), the tensile elongation is reduced for polymer–GO/ZrP composites [45].
Water contact angle
The surface wettability of a polyelectrolyte membrane indicates its tendency to swell or absorb water, which is related to IEC, water stability, and proton conductivity of the prepared membranes. As a result, the water contact angle was utilized to analyze this property. A lower contact angle, in general, indicates a more hydrophilic membrane surface [46].
Contact angle measurements of pristine PVC and PVC/GO composite membranes are shown in Table 8. As the GO concentration in the polymer matrix elevated, the contact angle decreased, suggesting that the hydrophilicity of PVC/GO hybrid membranes enhanced dramatically. Furthermore, water dropped on modified membranes permeated the membranes faster than water dropped on pristine PVC membranes. This is because of including GO with a significant number of hydrophilic functional groups (i.e., -O.H. and -COOH groups) [47].
Contact angle measurements of prepared PVC/GO-ZrP composite membranes are shown in Table 9. It can be seen that the hydrophilicity of the prepared membranes increases with increasing the quantity of zirconium phosphate. This is because zirconium phosphate contains phosphoric acid groups that increase the attraction of hybrid films to water molecules [48].
FT-IR Spectroscopy analysis
FT-IR spectra of Graphite and GO were presented in Fig. 7. It was observed that no significant peak was found in Graphite. Hence, the existence of carbonyl and carboxyl groups in the spectrum of GO suggested that Graphite was successfully oxidized into GO by the modified Hummers process [49, 50]. A prominent band at 3419.90 cm−1 was mainly related to (O–H) vibration, which considerably improves the wettability of GO [46]. There was a peak at 1730 cm−1, which is typical of carbonyl and/or carboxyl group vibration [51]. The 1629 cm−1 peak is connected with stretching vibration (C = C), which indicates the graphene structure. Moreover, the 1392 cm−1 peak is due to the bending vibration of (C–O–C), whereas the stretching vibration of (O = C-O) produced from the O–H group appeared at the 1220 cm−1 peak, reflecting the production of GO [52].
FT-IR spectra of ZrOCl2.8H2O and ZrP powder are shown in Fig. 8. The spectrum of ZrOCl2.8H2O powder shows that the band at 1623 cm−1 is attributed to the coordinated water "scissor" bending mode [53].
FT-IR spectral assignment of ZrP powder reveals the existence of distinctive peaks with ZrP (confirming ZrP production), leading to hydrogen bond formation at the P = O group. The 1639.55 cm−1 peaks were caused by the H–O-H. The two peaks remaining, at 511 cm−1 and 599 cm−1, were caused by the Zr-O and (PO4) groups, respectively. The peak at 1066.88 cm−1 was due to (P = O), and the 2378.31 cm−1 peak was caused by (POH). The peak at 3433.41 cm−1 was caused by -O.H. stretching and suggested the existence of water bound in ZrP [15, 54].
FT-IR spectral assignments of pristine PVC, PVC/GO, and PVC/GO-ZrP composite membranes are shown in Fig. 9. The spectrum of pristine PVC membrane exhibit a characteristic band at 2939.61 cm−1 due to the C–H stretching vibrations. The bands at 960 cm−1 and 1259.56 cm−1 are attributed to Trans CH wagging vibrations and C.H. rocking, respectively. The 1384.94 cm−1 and 1066 cm−1 bands are caused by stretching CH2 and C–C wagging, respectively. The C–Cl band stretching was found at 642 cm−1 [55].
FT-IR spectral assignment of PVC/GO composite membranes shows an increase in the peak intensity at 2923.82 cm−1, which is attributed to the C-H stretching vibrations of PVC. Also, a rise in the peak's amplitude was observed in the region 1000–1500 cm−1 with increasing the molar content of GO [56].
X-ray Diffraction (XRD) patterns
Natural Graphite and GO were analyzed by XRD patterns, as shown in Fig. 10. depicts the patterns of XRD used to characterize the crystalline nature and phase purity of the Graphite and a synthesized GO In the case of pristine Graphite, strong diffraction at (2θ = 26.4°) was observed, which is related to the (002) plane hexagonal lattice [57]. The equivalent interlayer distance was 0.33 nm.
The oxidation of Graphite into GO increased interplanar space. This is connected to the creation of interpenetrating groups containing oxygen. A broad peak for GO, at 2θ = 10.8° due to the basal plane (001), shows intercalated different oxygen-containing functional groups and H2O molecules [58]. The equivalent gap between layers was 0.88 nm.
The increasing gap between layers from 0.33 nm to 0.88 nm gives rise to shifting the 2θ peak shift from 26° to 10.8°, indicating that Graphite has been fully oxidized [59]. The gap between layers elevates due to the intercalation of different oxygen-containing functional groups and water molecules [60].
XRD patterns of pristine PVC, PVC/GO, and PVC/GO-ZrP composite membranes are shown in Fig. 11. It was observed that the amorphous phase of pristine PVC by detecting a distinct peak at about (2θ = 23°). The resulting PVC/GO, and PVC/GO-ZrP composite membranes have the same diffraction peaks as virgin PVC, according to XRD patterns. However, crystalline regions of PVC were suppressed by increasing the portion of GO in the blend membranes. These findings can be explained by the probable interactions between the membrane-forming components, which could prevent the polymer chains from stacking [61].
Morphological and microstructure features
Morphological features of the surface of PVC, PVC/GO, and PVC/GO-ZrP membranes were used to examine the microstructure of the fabricated membranes. SEM micrographs of prepared PVC/GO composite membranes with varying GO content (0.5, 1, and 1.5%) were shown in Fig. 12, compared to pristine PVC, which illustrates the influence of GO incorporation on membrane surface morphology. In general, the integration of GO substantially influences the characteristics of PVC, even at a modest percentage of added GO flakes [62]. It was observed that the surface of incorporating GO with PVC becomes rougher than a pure PVC membrane surface. On the surface of modified PVC membranes, a pore-like structure may be noticed in SEM pictures, which was strengthened by the inclusion of GO, which can decrease the stability of thermodynamics of PVC polymer solution and, therefore, considerably aid membrane development by enabling phase inversion [63]. The potential motive is that the PVC solution with large viscosity, loaded with GO (1.5%), acts as a physical impediment to solvent and non-solvent mass transfer, slowing and inhibiting the growth of macro-voids in the PVC/GO films. This is reliable to previous research on various nanoparticles, such as ZnO, Ag, TiO2, and SiO2, which have were published in the literature [64, 65]. The researchers discovered that increasing the GO loading induces a surface modification morphology of the prepared films, exposing an interconnected network of macro-pores.
In addition, SEM micrographs of PVC/GO composites membrane indicate that the bulk of the GO is dispersed uniformly throughout the polymer matrix, with no agglomeration. Furthermore, because polymers form in the pores and galleries of GO, distinguishing the various phases of GO and PVC in PVC/GO composites is challenging [66, 67].
On the other side, morphological features of the PVC/GO-ZrP composite membrane are shown in Fig. 13. It was observed that ZrP molecules were filling the pores of the PVC/GO composite membrane. Furthermore, these micrographs revealed that these membranes have a more uniform and compact structure, as well as a rougher surface than their corresponding based on ZrP content.
As a result, SEM analysis showed that GO and ZrP created a homogeneous composite with PVC filling the gaps between the GO sheets, resulting in a microporous morphology [22].
Transmission Electron Microscope (TEM)
TEM micrographs of surface examinations of natural graphite and synthetic graphene oxide are shown in Figs. 14 and 15, respectively to indicate the morphological properties. As the material was manufactured in powder form, a good view of the images revealed that the samples have multiple layers.
After oxidation of purified natural flake graphite (NFG) using the Hummers method, the synthesized GO sample has a laminar and thin structure with a size of around 1 mm2. Meanwhile, a wrinkled structure in GO was found due to edge-to-edge contacts. This can potentially increase the surface area of GO [68]. Furthermore, the unique structure of GO may facilitate its interaction with PVC polymers, increasing the mechanical properties of hybrid membranes [69].
The morphology of graphene oxide showed thick flat wafer layers, rough surface, uneven shape, and irregular particle size. Since the oxygen atoms were not removed and there was a large degree of exfoliation during the oxidation process, the structure was turbulent and not wrinkled. Moreover, fracture of the structure occurred during the oxidation process with an unexpected temperature or oxidizing amount, resulting in uneven shape and disordered particle size [70].
The wrinkled shell structure is morphologically similar to the thin film of graphene sheets. Rapid removal of intercalated oxygen and other functional groups between the layers led to exfoliation of the reducing medium and sonication, resulting in multi-layered thin layers and wrinkled plates [71]. As a result of the exfoliation process, the solid graphene structure became disordered, resulting in the plates crunching. As a result of correlating the results of graphene morphology and structural properties at 2 = 26.4°, it has been demonstrated that there is little or no oxygen function in the graphene formation [72, 73].
Thermogravimetric analysis (TGA)
The outcome of the thermogravimetric analysis, as is widely known, is depicted as curves illustrating the thermal degradation of organic molecules against increasing temperature.
TGA analysis of pristine PVC and PVC/GO composite membranes is shown in Fig. 16. As can be seen, all membranes showed the same two-step decomposition pattern as pristine PVC films, with first-stage dissociation occurring between 200 and 300 °C, possibly due to loss of HCl (dehydrochlorination) [74]. In this temperature range, PVC dechlorination and loss of plasticizers and other additives may occur. This can be described as follows: With increasing temperature, Cl radicals from (-C–Cl) bonds accept hydrogen radicals from surrounding C-H groups to create a covalent bond (H-Cl), which leads to the liberation of HCl molecules and the development of double bonds along the PVC chain [75, 76].
The second stage reaches its apex at 495.2 °C due to events involving the breakage of (C-H) bonds and the fragmentation of the backbone.
On the other hand, first-stage dissociation of PVC/GO composite films was detected in the range of 200–300 °C, which might be attributed to the removal of oxygen-containing functional groups from GO. A second weight loss was seen in the temperature range of 300–500 °C, which could be attributed to the degradation of the alternative single and double carbon bonds, leading to the development of a carbonaceous residue [77]. This implies that PVC and GO have a significant interaction, leading to the composites' improved thermal stability.
TGA analysis of PVC/GO-ZrP composite membranes is shown in Fig. 17. As can be seen, the weight loss in the PVC/GO-ZrP membrane was caused by the loss of structurally bound water linked with ZrP particles. TGA results reveal the moisture retention capability of PVC/GO-ZrP membranes at greater temperatures than Nafion-117. ZrP particles demonstrated exceptional thermal stability and water-retaining capacity even at high temperatures. The degradation of the polymeric chain of the PVC support caused significant weight loss in the PVC/GO-ZrP membrane at 420 °C [78]. Finally, it was discovered that integrating GO and ZrP into the PVC matrix increases the thermal stability of the composites. It is also clear that modified membranes have sufficient thermal characteristics for use in DMFCs because thermal breakdown is found between 200–300 °C. A slight change at 100 °C is found for all membranes, which corresponds to the weight loss of absorbed water. It also observed that phosphate group thermal degradation induces the transition at about 250 °C, while main chain scission causes the change at 380 °C (Tables 10 and 11).
Conclusion
This study focuses on preparing PVC/GO and PVC/GO-ZrP composite membranes using variable ratios of PVC, GO, and ZrP to optimize the performance and membrane properties in DMFCs operation. The structural and functional properties of prepared PVC and its blend-based membranes were characterized. The results show that polyelectrolyte composite membranes based on PVC modified with GO were prepared using the phase inversion method to improve their mechanical properties and hydrophilicity by incorporating GO modified by the Hummer's method and displayed many hydrophilic groups on the surface. The influence of GO incorporation on hybrid membranes' shape, thermal stability, mechanical stability, and hydrophilicity was primarily studied. The fabricated PVC/GO, and PVC/GO-ZrP composite membranes exhibited high thermal stability, so they withstood the high temperature (200–300 °C) for thermal decomposition onset. The IEC value increased with increasing the proportion of GO and ZrP in the polymer blend. Ion exchange capacity reached its maximum (0.75 meq/g) in the case of PVC/GO-ZrP composite membranes using (3%) PVC, (1.5%) GO, and the highest level of (ZrOCl2).
Incorporation of the ZrP ion exchanger into the pores of the PVC/GO membrane increases the ion exchange capacity (IEC) of the membranes compared to the PVC/GO membranes, for example, the IEC values increased from 0.27 to 0.75 meq /g, when the PVC/GO composite films were immersed at different concentrations of ZrOCl2 solution (5 to 15 wt%), due to the increased content of ZrP on the membrane surface.
The compositional and structural properties of prepared PVC/GO and PVC/GO-ZrP composite membranes were studied using FTIR, XRD, and SEM techniques. FT-IR spectra indicated the complete oxidation and conversion of Graphite into GO by indicating the characteristic peaks of carboxylic and carbonyl groups and the conversion of ZrOCl2.8H2O into ZrP by indicating the characteristic peaks of (P = O) group. XRD pattern indicated the conversion of natural Graphite to Go and indicated the presence of crystalline phase in the composite membranes. In summary, it was reasonable to conclude that the performance of PVC/GO composite membranes could be optimized by incorporating the ZrP appropriately to tune proton conduction and methanol barrier characteristics. In summary, it was plausible to deduce that the performance of PVC/GO composite membranes could be improved by effectively incorporating ZrP to improve proton conductivity and methanol barrier properties. As a result, these novel proton-conducting membranes are potential low-cost candidates for DMFC applications.
References
Nagar H, Sahu N, Rao VVB, Sridhar S (2020) Surface modification of sulfonated polyethersulfone membrane with polyaniline nanoparticles for application in a direct methanol fuel cell. Renew Energy 146:1262–1277. https://doi.org/10.1016/j.renene.2019.06.175
Abu-Saied MA, El-Desouky EA, Soliman EA, El-Naim GA (2020) Novel sulphonated poly (vinyl chloride)/poly (2-acrylamide-2-methylpropane sulphonic acid) blends-based polyelectrolyte membranes for direct methanol fuel cells. Polym Test 89(Sep 2020):106604. https://doi.org/10.1016/j.polymertesting.2020.106604
Garraín D, Lechón Y, Rúa C (2011) Polymer Electrolyte Membrane Fuel Cells (PEMFC) in automotive applications: environmental relevance of the manufacturing stage. Smart Grid Renew Energy 2(2). https://doi.org/10.4236/sgre.2011.22009
Jorissen L, Gogel V, Kerres J, Garche J (2002) New membranes for direct methanol fuel cells. J Power Sources 105(2):267–273. https://doi.org/10.1016/S0378-7753(01)00952-1
Tezuka T, Tadanaga K, Matsuda A, Hayashi A, Tatsumisago M (2005) Utilization of glass papers as support for proton conducting inorganic-organic hybrid membranes from 3-glycidoxypropyltrimethoxysilane. Electrochem Commun 7(3):245–248. https://doi.org/10.1016/j.elecom.2005.01.009
Rhim J-W, Park HB, Lee C-S, Jun J-H, Kim DS, Lee YM (2004) Crosslinked poly (vinyl alcohol) membranes containing sulfonic acid group: proton and methanol transport through membranes. J Membrane Sci 238(1):143–151. https://doi.org/10.1016/j.memsci.2004.03.030
Nolte R, Ledjeff K, Bauer M, Mulhaupt R (1993) Partially sulfonated poly (arylene ether sulfone) - A versatile proton conducting membrane material for modern energy conversion technologies. J Memb Sci 83(2):211–220. https://doi.org/10.1016/0376-7388(93)85268-2
Yoshikawa M, Tsubouchi K, Guiver MD, Robertson GP (1999) Modified polysulfone membranes. III. Pervaporation separation of benzene-cyclohexane mixtures through carboxylated polysulfone membranes. J Appl Polym Sci 74(2):407–412
Duangkaew P, Wootthikanokkhan J (2008) Methanol permeability and proton conductivity of direct methanol fuel cell membranes based on sulfonated poly (vinyl alcohol)-layered silicate nanocomposites. J Appl Polym Sci 109:452–458. https://doi.org/10.1002/app.28072
Helen M, Viswanathan B, Srinivasa Murthy S (2006) Fabrication and properties of hybrid membranes based on salts of heteropolyacid, zirconium phosphate and polyvinyl alcohol. J Power Sources 163(1):433–439
Alberti G, Casciola M, Capitani D, Donnadio A, Narducci R, Pica M, Sganappa M (2007) Novel Nafion-zirconium phosphate nanocomposite membranes with enhanced stability of proton conductivity at medium temperature and high relative humidity. Electrochim Acta 52(28):8125–8132. https://doi.org/10.1016/j.electacta.2007.07.019
Thakkar R, Chudasama U (2009) Synthesis and characterization of zirconium titanium phosphate and its application in separation of metal ions. J Hazard Mater 172(1):129–137
Nagarale RK, Shin W, Singh PK (2010) Progress in ionic organic-inorganic composite membranes for fuel cell applications. Polym Chem 1(4):388–408
Alberti G, Casciola M, Palombari R (2000) Inorganic-organic proton conducting membranes for fuel cells and sensors at medium temperatures. J Memb Sci 172(1–2):233–239
Pandey J, Seepana MM, Shukla A (2015) Zirconium phosphate based proton conducting membrane for DMFC application. Int J Hydrogen Energy 40(30):9410–9421
Walther Gustav Grot, Chadds Ford Pa., Govindarajulu Rajendran, Newark, Del. (1997) Membranes containing inorganic fillers and membrane and electrode assemblies and electrochemical cells employing same. United States patent [US5919583A].
Apichatachutapan W, Moore RB, Mauritz KA (1996) Asymmetric Nafion/ (zirconium oxide) hybrid membranes via in situ sol-gel chemistry. J Appl Polym Sci 62(2):417–426
Clearfield A (1988) Role of ion exchange in solid-state chemistry. Chem Rev 88(1):125–148. https://doi.org/10.1021/cr00083a007
Huang K, Liu G, Lou Y, Dong Z, Shen J, Jin W (2014) A graphene oxide membrane with highly selective molecular separation of aqueous organic solution. Angew Chem Int Ed 53(27):6929–6932
Paneri A, Heo Y, Ehlert G, Cottrill A, Sodano H, Pintauro P, Moghaddam S (2014) Proton selective ionic graphene-based membrane for high concentration direct methanol fuel cells. J Memb Sci 467:217–225. https://doi.org/10.1016/j.memsci.2014.05.002
Ahmad N, Kausar A, Muhammad B (2016) An investigation on 4-aminobenzoic acid-modified polyvinyl chloride/graphene oxide and PVC/graphene oxide based nanocomposite membranes. J Plast Film Sheeting 32(4):419–448
Deshmukh K, Joshi GM (2014) Thermo-mechanical properties of poly (vinyl chloride)/graphene oxide as high-performance nanocomposites. Polym Test 34:211–219
Metrangolo P, Meyer F, Pilati T, Resnati G, Terraneo G (2008) Halogen bonding in supramolecular chemistry. Angew Chem Int Ed 47(33):6114–6127
Salavagiane HJ, Miras MC, Barbero C (2006) Photolithographic patterning of a conductive polymer using a polymeric photoacid generator and a traceless removable group. Macromol Rapid Commun 27(1):26–30
Salavagione HJ, Martínez G (2011) Importance of covalent linkages in the preparation of effective reduced graphene oxide-poly(vinyl chloride) nanocomposites. Macromolecules 44(8):2685–2692
Zhang L, Mukherjee S (2006) Investigation of durability issues of selected non-fluorinated polymer exchange membranes for fuel cell applications. J Electrochem Soc 153:A1062–A1072. https://doi.org/10.1149/1.2180715
Muthumeenal A, Neelakandan S, Kanagaraj P, Nagendran A (2016) Synthesis and properties of novel proton exchange membranes based on sulfonated polyethersulfone and N-phthaloyl chitosan blends for DMFC applications. Renew Energy 86:922–929
Guo C, Zhou L, Lv J (2013) Effects of expandable Graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym Polym Compos 21(7):449–456
Vinodh R, Ilakkiya A, Elamathi S, Sangeetha D (2010) A novel anion exchange membrane from polystyrene (ethylene butylene) polystyrene: synthesis and characterization. Mater Sci Eng B Solid-State Mater Adv Technol 167(1):43–50
Zainoodin AM, Kamarudin SK, Masdar MS, Daud WRW, Mohamad AB, Sahari J (2014) High power direct methanol fuel cell with a porous carbon nanofiber anode layer. Appl Energy 113:946–954
Kamoun EA, Abu-Saied M, Doma A, Menzel H, Chen X (2018) Influence of degree of substitution and folic acid coinitiator on pullulan-HEMA hydrogel properties crosslinked under visible-light initiating system. Int J Biol Macromol 116:1175–1185
Hasan M, Banerjee AN, Lee M (2015) Enhanced thermo-optical performance and high BET surface area of graphene-PVC nanocomposite fibers prepared by simple facile deposition technique: N2 adsorption study. J Ind Eng Chem 21:828–834
Abu-Saied M, Khalil KA, Al-Deyab SS (2012) Preparation and characterization of polyvinyl acetate nanofiber doping copper metal. Int J Electrochem Sci 7:2019–2027
Akita T, Taniguchi A, Maekawa J, Siroma Z, Tanaka K, Kohyama M, Yasuda K (2006) Analytical TEM study of Pt particle deposition in the proton-exchange membrane of a membrane-electrode-assembly. J Power Sources 159(1):461–467. https://doi.org/10.1016/j.jpowsour.2005.10.111
Dicks A, Rand DAJ (2018) Fuel cell systems explained. Wiley Online Library. https://doi.org/10.1002/9781118706992
Becker W, Schmidt-Naake G (2002) Proton exchange membranes by irradiation-induced grafting of styrene onto FEP and ETFE: Influences of the crosslinker N, N,-Methylene-bis-acrylamide. Chem Eng Technol 25(4):373–377
Paneri A et al (2014) Proton selective ionic graphene-based membrane for high concentration direct methanol fuel cells. J Memb Sci 467:217–225. https://doi.org/10.1016/j.memsci.2014.05.002
Commer P, Cherstvy AG, Spohr E, Kornyshev AA (2002) The effect of water content on proton transport in polymer electrolyte membranes. Fuel Cells 2(34):127–136
Peighambardoust J, Rowshanzamir S, Amjadi M (2010) Review of the proton exchange membranes for fuel cell applications. I J Hydrogen 35(17):9349–9384. https://doi.org/10.1016/j.ijhydene.2010.05.017
Kuijt J, Ariese F, Brinkman UAT, Gooijer C (2003) Room temperature phosphorescence in the liquid state as a tool in analytical chemistry. Anal Chim Acta 488(2):135–171
Brostow W, HaggLobland HE (2017) Materials: Introduction and Applications. Wiley
Menges G, Boden H-E (1986) In: Brostow W, Corneliussen RD (eds) Failure of plastics. Hanser Publishers, Munich
Brostow W, Hagg Lobland HE, Narkis M (2006) Sliding wear, viscoelasticity, and brittleness of polymers. J Mater Res 21(9):2422–2428. https://doi.org/10.1557/jmr.2006.0300
Wang X, Li Y, Liao W, Gu J, Li D (2008) A new intumescent flame-retardant: preparation, surface modification, and its application in polypropylene. Polym Advan Technol 19:1055–1061. https://doi.org/10.1002/pat.1077
Brostow W, Hagg Lobland HE (2010) Brittleness of materials: Implications for composites and a relation to impact strength. J Mater Sci 45(1):242–250. https://doi.org/10.1007/s10853-009-3926-5
Vatanpour V, Madaeni SS, Moradian R, Zinadini S, Astinchap B (2012) Novel anti-biofouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes. Sep Purif Technol 90:69–82
Eldin MSM, Elzatahry AA, El-Khatib KM, Hassan EA, El-Sabbah MM, Abu-Saied MA (2010) Novel grafted nafion membranes for proton-exchange membrane fuel cell applications. J Appl Polym Sci 119:120–133. https://doi.org/10.1002/app.32613
Yeole N, Kutcherlapati SNR, Jana T (2015) Polystyrene-graphene oxide (GO) nanocomposite synthesized by interfacial interactions between RAFT modified GO and core-shell polymeric nanoparticles. J Colloid Interface Sci 443:137–142. https://doi.org/10.1016/j.jcis.2014.11.071
Choi E, Han TH, Hong J, Kim JE, Lee SH (2010) noncovalent functionalization of graphene with end-functional polymers. J Mater Chem 20(10):1907–1912. https://doi.org/10.1039/B919074K
Chen X, Lai X, Hu J, Wan L (2015) An easy and novel approach to prepare Fe 3 O 4 –reduced graphene oxide composite and its application for high-performance lithium-ion batteries. RSC Adv 5(77):62913–62920
Yin G, Zheng Z, Wang H, Du Q, Zhang H (2013) Preparation of graphene oxide coated polystyrene microspheres by Pickering emulsion polymerization. J Colloid Interface Sci 394:192–198. https://doi.org/10.1016/j.jcis.2012.11.024
Zinadini S, Zinatizadeh AA, Rahimi M, Vatanpour V, Zangeneh H (2014) Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J Memb Sci 453:292–301
Yan C, Liu R, Zhang C, Cao Y (2016) Entirely aqueous solution-gel route for the preparation of zirconium carbide, hafnium carbide, and their ternary carbide powders. Ceram Silikaty 60(3):248–253
Helen M, Viswanathan B, Murthy SS (2007) Synthesis and characterization of composite membranes based on α-zirconium phosphate and silicotungstic acid. J Memb Sci 292(1–2):98–105
Ramesh S, Chai MF (2007) Conductivity, dielectric behavior and FTIR studies of high molecular weight poly(vinyl chloride)–lithium triflate polymer electrolytes. Mater Sci Eng B 139(2–3):240–245. https://doi.org/10.1016/j.mseb.2007.03.003
Zhao Y, Lu J, Liu X, Wang Y, Lin J, Peng N, Li J, Zhao F (2016) Performance enhancement of polyvinyl chloride ultrafiltration membrane modified with graphene oxide. J Colloid Interface Sci 480:1–8. https://doi.org/10.1016/j.jcis.2016.06.075
Bourlinos AB, Gournis D, Petridis D, Szabo T, Szeri A, Dekany I (2003) Graphite oxide: chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 19:6050–6055. https://doi.org/10.1021/la026525h
Jeong HK, Lee YP, Jin MH, Kim ES, Bae JJ, Lee YH (2009) Thermal stability of graphite oxide. Chem Phys Lett 470:255–258
Wang G, Yang J, Park J, Gou X, Wang B, Liu H, Yao J (2008) Facile synthesis and characterization of graphene nanosheets. J Phys Chem C 112:8192–8195. https://doi.org/10.1021/jp710931h
Park S, An J, Potts JR, Nelamakanni A, Murali S, Ruoff RS (2011) Hydrazine-reduction of Graphite- and graphene oxide. Carbon 49(9):3019–3023. https://doi.org/10.1016/j.carbon.2011.02.071
Ansari S, Giannelis EP (2009) Functionalized graphene sheet-poly (vinylidene fluoride) conductive nanocomposites. J Polym Sci Part B Polym Phys 47:888–897. https://doi.org/10.1002/polb.21695
Liu B, Chen C, Zhang W, Crittenden J, Chen Y (2012) Low-cost antifouling PVC ultrafiltration membrane fabrication with Pluronic F 127: effect of additives on properties and performance. Desalination 307:26–33. https://doi.org/10.1016/j.desal.2012.07.036
Garcia-Ivars J, Iborra-Clar MI, Alcaina-Miranda MI, Van der Bruggen B (2015) Comparison between hydrophilic and hydrophobic metal nanoparticles on the phase separation phenomena during the formation of asymmetric polyethersulphone membranes. J Memb Sci 493:709–722
Abu-Saied M, Fahmy A, Morgan N, Qutop W, Abdelbary H, Friedrich FJ (2019) Enhancement of poly(vinyl chloride) electrolyte membrane by its exposure to an atmospheric dielectric barrier discharge followed by grafting with polyacrylic acid. Plasma Chem Plasma Process 39(6):1499–1517. https://doi.org/10.1007/s11090-019-10017-6
Balta S, Sotto A, Luis P, Benea L, Van der Bruggen B, Kim J (2012) A new outlook on membrane enhancement with nanoparticles: the alternative of ZnO. J Memb Sci 389:155–161
Lin J, Ye W, Zhong K, Shen J, Jullok N, Sotto A, Van der Bruggen B (2016) Enhancement of polyethersulfone (PES) membrane doped by monodisperse Stöber silica for water treatment. Chem Eng Process Process Intensif 107:194–205. https://doi.org/10.1016/j.cep.2015.03.011
Konwer S, Guha AK, Dolui SK (2013) Graphene oxide-filled conducting polyaniline composites as methanol-sensing materials. J Mater Sci 48(4):1729–1739
Chen T, Xia Y, Jia Z, Liu Z, Zhang H (2014) Synthesis, characterization, and tribological behavior of oleic acid capped graphene oxide. J Nanomater. https://doi.org/10.1155/2014/654145
Song M, Yu L, Wu Y (2012) Simple synthesis and enhanced performance of graphene oxide-gold composites. J Nanomater. https://doi.org/10.1155/2012/135138
Oh WC, Zhang FJ (2011) Preparation and characterization of graphene oxide reduced from a mild chemical method. Asian J Chem 23(2):875–879. https://doi.org/10.1155/2014/276143
Li X, Wang Z, Wu L, Tsai T (2016) One-step: In situ synthesis of a novel α-zirconium phosphate/graphene oxide hybrid and its application in phenolic foam with enhanced mechanical strength, flame retardancy, and thermal stability. RSC Adv 6(78):74903–74912
Zhang L, Yu Z, Li D, Fan L, Zhu Y, Hong R, Hu Y, Fan J, Cen K (2013) Enhanced critical heat flux during quenching of extremely dilute aqueous colloidal suspensions with graphene oxide nanosheets. J Heat Transfer 135(5):54502. https://doi.org/10.1115/1.4023304
Shakshooki SK, EL-Tarhuni SR, EL-Hamady AM (2014) Synthesis and characterization of pellicular γ -zirconium phosphate fibrous cerium phosphate nanocomposite membranes. Am J Chem 4(4):109–115. https://doi.org/10.5923/j.chemistry.20140404.01
Vadukumpully S, Paul J, Mohanta N, Valiyaveettil S (2011) Flexible conductive graphene/poly(vinyl chloride) composite thin films with high mechanical strength and thermal stability. Carbon 49:198–205
Djidjelli H, Martinez-Vega JJ, Farenc J, Benachour D (2002) Effect of wood flour content on the thermal, mechanical and dielectric properties of poly(vinyl chloride). Macromol Mater Eng 287:611–618. https://doi.org/10.1002/1439-2054(20020901)287:9%3c611::AIDMAME611%3e3.0.C.O.;2-L
Djidjelli H, Sadoun T, Benachour D (2000) Effect of plasticizer nature and content on the PVC stability and dielectric properties. J Appl Polym Sci 78(3):685–691. https://doi.org/10.1002/1097-4628(20001017)78:3%3c685::AID-APP250%3e3.0.C.O.;2-F
El-aassar MR, El-kady MF, Hassan HS, Al-deyab SS (2016) Synthesis and characterization of surface modified electrospun poly (acrylonitrile-co-styrene) nanofibers for dye decolorization. J Taiwan Inst Chem Eng 58:274–282. https://doi.org/10.1016/j.jtice.2015.05.042
Diao H, Yan F, Qiu L, Lu J, Lu X, Lin B, Li Q, Shang S, Liu W, Liu J (2010) High-performance crosslinked poly (2-acrylamide-2-methyl propane sulfonic acid)-based proton exchange membranes for fuel cells. Macromolecules 43(15):6398–6405. https://doi.org/10.1021/ma1010099
Inan TY, Doan H, Unveren EE, Eker E (2010) Sulfonated PEEK and fluorinated polymer-based blends for fuel cell applications: Investigation of the effect of type and molecular weight of the fluorinated polymers on the membrane’s properties. Int J Hydrogen Energy 35(21):12038–12053. https://doi.org/10.1016/j.ijhydene.2010.07.084
Eldin MSM, Elzatahry AA, El-Khatib KM, Hassan EA, El-Sabbah MM, Abu-Saied MA (2011) Novel grafted Nafion membranes for proton-exchange membrane fuel cell applications. J Appl Polym Sci 119(1):120–133. https://doi.org/10.1002/app.32613
Abu-Saied MA, Fontananova E, Drioli E, Mohy Eldin MS (2013) Sulphonated poly (glycidyl methacrylate) grafted cellophane membranes: novel application in polyelectrolyte membrane fuel cell (PEMFC). J Polymer Res 20(7). https://doi.org/10.1007/s10965-013-0187-4
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elerian, A.F., Abu-Saied, M.A., Abd-Elnaim, G.H. et al. Development of polymer electrolyte membrane based on poly(Vinyl Chloride)/graphene oxide modified with zirconium phosphate for fuel cell applications. J Polym Res 30, 6 (2023). https://doi.org/10.1007/s10965-022-03317-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03317-7