Abstract
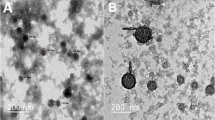
A method to obtain a multilayer insulin-containing composition in a nanostructured form consisting of layers: gold-chitosan-insulin-chitosan nanoparticles was developed. The size of the fully formed multicomponent system was 178 nm.The outer layer of chitosan protects insulin from the damaging effects of low pH-medium and proteolytic enzymes of the gastrointestinal tract when the composition is taken orally. Chitosan simultaneously performs a transport function to deliver insulin into the bloodstream. In experiments on experimental animals with artificially induced type 1 diabetes, it was found that the effectiveness and duration of hypoglycemic action of the composition depends on the amount of insulin in the dose. The composition containing 50 IU of insulin per dose provides a rapid decrease glucose level in the blood and maintains it at the level of the intact group for 3 h. After 6 h the efficacy of the composition is comparable to that of insulin injection. A fundamentally important indicator of the effectiveness of the composition is a constant content of glycated hemoglobin in the blood within 10 days.









Similar content being viewed by others
References
Gorska-Ciebiada M, Masierek M, Ciebiada M (2020) Improved insulin injection technique, treatment satisfaction, and glycemic control: Results from a large cohort education study. J Clin Transl Endocrinol 19:100217. https://doi.org/10.1016/j.jcte.2020.100217
Pan Y, Li YJ, Zhao HY, Zheng JM, Xu H, Wei G, Hao JS, Cui F (2002) Bioadhesive polysaccharides in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm 249:139–147. https://doi.org/10.1016/j.jcte.2020.100217
Makhlof A, Tozuka Y, Takeuchi H (2011) Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur J Pharm Sci 42:445–451. https://doi.org/10.1016/j.ejps.2010.12.007
Yin L, Ding J, He C, Cui L, Tang C, Yin C (2009) Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomater 30:5691–5700. https://doi.org/10.1016/j.biomaterials.2009.06.055
Sedyakina N, Kuskov A, Velonia K, Feldman N, Lutsenko S, Avramenko G (2020) Modulation of entrapment efficiency and in vitro release properties of BSA-loaded chitosan microparticles cross-linked with citric acid as a potential protein–drug delivery system. Mater 13(8):1989. https://doi.org/10.3390/ma13081989
Bhumkar D, Joshi H, Sastry M, Pokharkar V (2007) Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res 24(8):1415–1426. https://doi.org/10.1007/s11095-007-9257-9
Jintapattanakit A, Junyaprasert V, Mao S, Sitterberg J, Bakowsky U, Kissel T (2007) Peroral delivery of insulin using chitosan derivatives: A comparative study of polyelectrolyte nanocomplexes and nanoparticles. Int J Pharm 342:240–249. https://doi.org/10.1016/j.ijpharm.2007.05.015
Chalasani KB, Russell-Jones GJ, Jain AK, Diwan PV, Jain SK (2007) Effective oral delivery of insulin in animal models using vitamin B12-coated dextran nanoparticles. J Control Release 122:141–150. https://doi.org/10.1016/j.jconrel.2007.05.019
Chalasani KB, Russell-Jones GJ, Yandrapu SK, Diwan PV, Jain SK (2007) A novel vitamin B12-nanosphere conjugate carrier system for peroral delivery of insulin. J Control Release 117(3):421–429. https://doi.org/10.1016/j.jconrel.2006.12.003
Sarmento B, Ribeiro A, Veiga F, Ferreira D (2006) Development and characterization of new insulin-containing polysaccharide nanoparticles. Colloids Surf B Biointerfaces 53:193–202. https://doi.org/10.1016/j.colsurfb.2006.09.012
Sarmento B, Ribeiro AJ, Veiga F, Ferreira DC, Neufeld RJ (2007) Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J Nanosci Nanotechnol 7(8):2833–2841. https://doi.org/10.1166/jnn.2007.609
Woitiski B, Neufeld RJ, Veiga F, Carvalho RA, Figueiredo IV (2010) Pharmacological effect of orally delivered insulin facilitated by multilayered stable nanoparticles. Eur J Pharm Sci 41:556–563. https://doi.org/10.1016/j.ejps.2010.08.009
Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, Hsu CW, Lin KJ, Sung HW (2010) Enteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomater 31:3384–3394. https://doi.org/10.1016/j.biomaterials.2010.01.042
Han L, Zhao Y, Yin L, Li R, Liang Y, Huang H, Pan S, Wu C, Feng M (2012) Insulin-loaded pH-sensitive hyaluronic acid nanoparticles enhance transcellular delivery. AAPS PharmSciTech 13:836–845. https://doi.org/10.1208/s12249-012-9807-2
Fonte P, Araújo F, Silva C, Pereirad C, Reisa S, Santos HA, Sarmento B (2015) Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol Adv 33(6/3):1342–1354. https://doi.org/10.1016/j.biotechadv.2015.02.010
Cheung RCF, Ng TB, Wong JH, Chan WY (2015) Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar Drugs 13(8):5156–5186. https://doi.org/10.3390/md13085156
Varlamov VP, Il’ina AV, Shagdarova BT, Lunkov AP, Mysyakina IS (2020) Chitin/Chitosan, and Its Derivatives: Fundamental Problems and Practical Approaches. Biochem(Moscow) 85:154–176. https://doi.org/10.1134/S0006297920140084
Moran HBT, Turley JL, Andersson M, Lavelle EC (2018) Immunomodulatory properties of chitosan polymers. Biomater 184:1–9. https://doi.org/10.1016/j.biomaterials.2018.08.054
Sogias IA, Williams AC, Khutoryanskiy VV (2008) Why is chitosan mucoadhesive? Biomacromol 9(7):1837–1842. https://doi.org/10.1021/bm800276d
Sonaje K, Chuang EY, Lin KJ, Yen TC, Su FY, Tseng MT, Sung HW (2012) Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: microscopic, ultrastructural, and computed-tomographic observations. Mol Pharm 9(5):1271–1279. https://doi.org/10.1021/mp200572t
Pechenkin MA, Balabushevich NG, Zorov IN, Staroseltseva LK, Mikhalchik EV, Izumrudov VA, Larionova NI (2011) Design, in vitro and in vivo characterization of chitosan – dextran sulfate microparticles for oral delivery of insulin. J Bioequiv Availab 3(10):244–250. https://doi.org/10.4172/jbb.1000094
Larionova NI, Zubaerova DK, Guranda DT, Pechyonkin MA, Balabushevich NG (2009) Colorimetric assay of chitosan in presence of proteins and polyelectrolytes by using ophthalaldehyde. Carbohydr Polym 75(4):724–727. https://doi.org/10.1016/j.carbpol.2008.10.009
Balabushevich NG, Pechenkin MA, Zorov IN, Shibanova ED, Larionova NI (2011) Mucoadhesive polyelectrolyte microparticles containing recombinant human insulin and its analogs aspart and lispro. Biochem(Moscow) 76(3):327–331. https://doi.org/10.1134/S0006297911030059
Balabushevich N, Pechenkin M, Shibanova E, Volodkin D, Mikhalchik E (2013) Multifunctional polyelectrolyte microparticles for oral insulin delivery. Macromol Biosci 13(10):1379–1388. https://doi.org/10.1002/mabi.201300207
He Z, Santos JL, Tian H, Huang H, Hu Y, Liu L, Leong KW, Chen Y, Mao HQ (2017) Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomater 130:28–41. https://doi.org/10.1016/j.biomaterials.2017.03.028
Mukhopadhyay P, Chakraborty S, Bhattacharya S, Mishra R, Kundu PP (2015) pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int J Biol Macromol 72:640–648. https://doi.org/10.1016/j.ijbiomac.2014.08.040
Finotelli PR, Silva D, Sola-Penna M, Rossi AL, Farina M, Andrade LR, Takeuchi AY, Rocha-Leão MH (2010) Microcapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulin. Colloids Surf B Biointerfaces 81:206–211. https://doi.org/10.1016/j.colsurfb.2010.07.008
Dykman L, Khlebtsov N (2012) Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev 41:2256–2282. https://doi.org/10.1039/C1CS15166E
Khlebtsov NG, Dykman LA (2011) Biodistribution and toxicity of gold nanoparticles. Nanotechnol Russ 6(1–2):17–42. https://doi.org/10.1134/S1995078011010101
Mochalova AE, Koryagin AS, Salomatina EV, Apryatina KV, Smirnova LA (2014) Chitosan-Coated Gold Nanoparticles as Promising Nanosystem for Directional Drug Delivery to Target Organs. J Nanotech Diagn Treat 2:34–41. https://doi.org/10.12974/2311-8792.2014.02.02.3
Glazova I, Smirnova L, Zamyshlyayeva O, Zaitsev S, Avdoshin A, Naumov V, Ignatov S (2019) Interpolymer interaction in insulin-chitosan complexes. Supramol Chem 31(6):412–423. https://doi.org/10.1080/10610278.2019.1614180
Koryagin AS, Mochalova AE, Salomatina EV, Eshkova OYU, Smirnova LA (2013) Adaptogenic effects of chitosan-gold nanocomposites under simulated hypoxic conditions. Inorg Mater Appl Res 4:127–130. https://doi.org/10.1134/S2075113313020081
Smirnova LA, Gracheva TA, Mochalova AE, Kuz’micheva TA, Fedoseeva EN (2010) Peculiarities of gold nanoparticle formation in chitosan solutions doped with HAuCl4. Nanotechnol Russ 5(1–2):78–82. https://doi.org/10.1134/s1995078010010088
Nieto-Suarez M, Vila-Romeu N, Prieto I (2008) Behavior of insulin Langmuir monolayers at the air–water interface under various conditions. Thin Solid Films 516(24):8873–8879. https://doi.org/10.1016/j.tsf.2007.11.062
Tarazona NA, Machatschek R, Schulz B, Prieto MA, Lendlein A (2019) Molecular insights into the physical adsorption of amphiphilic protein PhaF onto copolyester surfaces. Biomacromol 20(9):3242–3252. https://doi.org/10.1021/acs.biomac.9b00069
Soenen SJ, Manshian B, Montenegro JM, Amin F, Meermann B, Thiron T, Cornelissen M, Vanhaecke F, Doak S, Parak WJ, De Smedt S, Braeckmans K (2012) Cytotoxic effects of gold nanoparticles: A multiparametric study. ACS Nano 6(7):5767–5783. https://doi.org/10.1021/nn301714n
Apryatina KV, Gribanova MV, Markin AV, Sologubov SS, Smirnova LA (2016) Silver nanoparticle–chitosan complexes and properties of their composites. Nanotechnol Russ 11:766–775. https://doi.org/10.1134/S1995078016060033
Gracheva TA, Kuz’micheva TA, Perevezentsev VN, Smirnova LA, Mochalova AE, Salomatina EV (2015) Peculiarities of the kinetics of UV-induced formation of gold nanoparticles in chitosan solutions doped with HAuCl4. Tech Phys Lett 41:235–237. https://doi.org/10.1134/S1063785015030062
Nguyen HH, Brûlet A, Goudounèche D, Saint-Aguet P, Lauth-de Viguerie N, Marty JD (2015) The effect of polymer branching and average molar mass on the formation, stabilization and thermoresponsive properties of gold nanohybrids stabilized by poly(N-isopropylacrylamides). Polym Chem 6(32):5838–5850. https://doi.org/10.1039/C5PY00659G
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum–size–related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346. https://doi.org/10.1021/cr030698+
Link S, El-Sayed MA (1999) Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phys Chem B 103(21):4212–4217. https://doi.org/10.1021/jp984796o
Fong KE, Yung LYL (2013) Localized surface plasmon resonance: a unique property of plasmonic nanoparticles for nucleic acid detection. Nanoscale 5:12043–12071. https://doi.org/10.1039/C3NR02257A
Hunter RJ (1981) Zeta potential in colloid science. Academic Press, New York
Acknowledgements
This work was supported by a grant from the Russian Science Foundation No. 21-73-00188 and partly financial support via State assignment in the Research scientific laboratory of "Chemistry of natural products and their synthetic analogues" of Scientific Educational Centre "Technoplatform 2035".
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Conceptualization: [LA Smirnova], Data curation: [AS Koryagin], Writing – original draft: [IA Glazova], Project administration: [SD Zaitsev], Writing – review & editing [KV Apryatina].
Corresponding author
Ethics declarations
Ethics approval
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Conflicts of interest
All authors confirm that there are no known conflicts of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Apryatina, K.V., Glazova, I.A., Koryagin, A.S. et al. Multilayer nanostructured system for oral insulin delivery. J Polym Res 29, 378 (2022). https://doi.org/10.1007/s10965-022-03225-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03225-w