Abstract
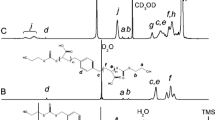
Polymerization kinetics as well as thermal properties of acrylic copolymers containing Isobornylacrylate- (IBOA) and 2-Ethylhexylacrylate- (2-EHA) units were investigated. Poly(IBOA-co-2-EHA) samples were synthesized via free radical photopolymerization/crosslinking reactions of IBOA and 2-EHA, in the presence of 1,6-hexanedioldiacrylate (HDDA) as crosslinking agent, to obtain chemically crosslinked polymer networks. High conversion rates of the acrylic double bonds of the monomers were obtained from investigation of the polymerization kinetics by infrared spectroscopy. Analysis of the thermal properties using differential scanning calorimetry revealed the appearance of a single glass transition of Poly(IBOA-co-2-EHA) over a large range of temperatures comprised between 208 and 321 K, depending on monomer composition. The evolution of the glass transition temperature was rationalized by applying the Fox, Gordon Taylor, and Brekner-Schneider-Cantow models, revealing presumably the existence of hydrogen bonding interaction involving the carbonyl groups of the acrylates. Several degradation processes were observed by thermogravimetrical analysis, especially that of the isobornylene group at low temperature followed by the degradation of the carbon backbone at higher temperatures. Increasing IBOA content leads to a higher thermal stability of Poly(IBOA-co-2-EHA). Each degradation step could be characterized separately exhibiting activation energies which strongly depend on the degradation time of each step.









Similar content being viewed by others
References
Brandrup J, Immergut EH, Grulke EA (2003) Polymer Handbook. John Wiley & Sons, Chichester, West Sussex, UK
Shrivastava A (2018) Introduction to Plastics Engineering. Elsevier, Amsterdam, Netherlands. https://doi.org/10.1016/C2014-0-03688-X
Zhang C, Bai Y, Cheng B, Liu W (2018) Adhesion properties of atactic polypropylene/acrylate blend copolymer and its adhesion mechanism for untreated polypropylene materials. Int J Adhes Adhes 80:7–15. https://doi.org/10.1016/j.ijadhadh.2017.09.007
Wu WC, Wang DM, Lin YC, Dai CA, Cheng KC, Hu MS, Lee BS (2016) Hydrogen bonds of a novel resin cement contribute to high adhesion strength to human dentin. Dent Mater 32:114–124. https://doi.org/10.1016/j.dental.2015.11.002
Richter A (2010) Hydrogels for actuators. Springer series on chemical sensors and biosensors, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-75645-3_7
Voit W, Ware T, Gall K (2010) Radiation crosslinked shape-memory polymers. Polymer (Guildf) 51:3551–3559. https://doi.org/10.1016/j.polymer.2010.05.049
Richard RE, Schwarz M, Ranade S, Chan AK, Matyjaszewski K, Sumerlin B (2005) Evaluation of acrylate-based block copolymers prepared by atom transfer radical polymerization as matrices for paclitaxel delivery from coronary stents. Biomacromol 6:3410–3418. https://doi.org/10.1021/bm050464v
López-García MdC, Beebe DJ, Crone WC (2007) Characterization of Poly(isobornyl acrylate) as a Construction Material for Microfluidic Applications. J Appl Polym Sci 21:449–456. https://doi.org/10.1002/app.26195
Roose P (2013) Residual stress in radiation-cured acrylate coatings. React Funct Polym 73:323–331. https://doi.org/10.1016/j.reactfunctpolym.2012.05.002
Zeggai N, Dali Youcef B, Dubois F, Bouchaour T (2018) Analysis of dynamic mechanical properties of photochemically crosslinked poly(isobornylacrylate-co-isobutylacrylate) applying WLF and Havriliak-Negami models. Polym Test 72:432–438. https://doi.org/10.1016/j.polymertesting.2018.10.038
Jakubowski W, Juhari A, Best A, Koynov K, Pakula T, Matyjaszewski K (2008) Comparison of thermomechanical properties of statistical, gradient and block copolymers of isobornyl acrylate and n-butyl acrylate with various acrylate homopolymers. Polymer 49:1567–1578. https://doi.org/10.1016/j.polymer.2008.01.047
Weng L, Vijayaraghavan R, MacFarlane DR, Elliott GD (2014) Application of the Kwei equation to model the Tg behavior of binary blends of sugars and salts. Cryobiology 68:155–158. https://doi.org/10.1016/j.cryobiol.2013.12.005
Appell M, Schmidt-Naake G (2004) Stable free-radical copolymerization of styrene with acrylates using OH-TEMPO. Macromol Chem Phys 205:637–644. https://doi.org/10.1002/macp.200300069
Kuo SW, Kao HC, Chang FC (2003) Thermal behavior and specific interaction in high glass transition temperature PMMA copolymer. Polymer 44:6873–6882. https://doi.org/10.1016/j.polymer.2003.08.026
(a) Flynn JH, Wall LA (1966) A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett 4:323–328. https://doi.org/10.1002/pol.1966.110040504. (b) Flynn JH, Wall LA (1966) General Treatment of the Thermogravimetry of Polymers. J Res Natl Bur Stand Sect A 70:487–523. https://nvlpubs.nist.gov/nistpubs/jres/70A/jresv70An6p487_A1b.pdf
Rouquerol J (1964) Etude de l’eau de constitution de plusieurs oxydes a grande surface specifique (glucine, alumine, silice-alumine). University of Paris, France, PhD-thesis
Paulik F, Paulik J (1971) Quasi-isothermal thermogravimetry. Anal Chim Acta 56:328–331. https://doi.org/10.1016/S0003-2670(01)82431-4
Abadie MJM, Popa M, Zaharia-arnautu M, Bulacovschi V (2000) Kinetics of photochemical polymerization of monomers acrylics in the presence of functionalized silica. Eur Polym J 36:571–581. https://doi.org/10.1016/S0014-3057(99)00097-X
Ye S, Cramer NB, Bowman CN (2011) Relationship between Glass Transition Temperature and Polymerization Temperature for Cross-Linked Photopolymers. Macromolecules 490–494. https://doi.org/10.1021/ma101296j
Sokolowski W, Metcalfe A, Hayashi S (2007) Medical applications of shape memory. Biomed Mater 2:S23. https://doi.org/10.1088/1748-6041/2/1/S04
Fox TG, Flory PJ (1954) The glass temperature and related properties of polystyrene. Influence of molecular weight. J Polym Sci 14:315–319. https://doi.org/10.1002/pol.1954.120147514
Gordon M, Taylor JS (1952) Ideal copolymers and the second-order transitions of synthetic rubbers. i. non-crystalline copolymers. J Appl Chem 2:493–500. https://doi.org/10.1002/jctb.5010020901
Brekner MJ, Schneider HA, Cantow HJ (1988) Approach to the composition dependence of the glass transition temperature of compatible polymer blends: 1. Polymer (Guildf) 29:78–85. https://doi.org/10.1016/0032-3861(88)90203-0
Zeggai N, Bouberka Z, Dubois F, Bouchaour T, Dali Youcef B, Delarace L, Potier J, Supiot P, Maschke U (2021) Effect of structure on the glass transition temperatures of linear and crosslinked poly(isobornylacrylate-coisobutylacrylate). J Appl Polym Sci 138:50449. https://doi.org/10.1002/app.50449
Haloi DJ, Singha NK (2011) Synthesis of poly(2-ethylhexyl acrylate)/clay nanocomposite by in situ living radical polymerization. J Polym Sci Part A Polym Chem 49:1564–1571. https://doi.org/10.1002/pola.24577
Ors JA, La Perriere DM (1986) Thermogravimetric profile of decomposition of acrylate systems based on bornyl acrylate monomers. Polymer 27:1999–2002. https://doi.org/10.1016/0032-3861(86)90197-7
Ozlem S, Aslan-Gürel E, Rossi RM, Hacaloglu J (2013) Thermal degradation of poly(isobornyl acrylate) and its copolymer with poly(methyl methacrylate) via pyrolysis mass spectrometry. J Anal Appl Pyrolysis 100:17–25. https://doi.org/10.1016/j.jaap.2012.10.024
Matsumoto A, Mizuta K, Otsu T (1993) Synthesis and thermal properties of poly(cycloalkyl methacrylate)s bearing bridged- and fused-ring structures. J Polym Sci Part A Polym Chem 31:2531–2539. https://doi.org/10.1002/pola.1993.080311014
Qu J, Cheng J, Wang Z, Han X, Zhao M (2014) Synthesis, thermal and optical properties of crosslinked poly(isobornyl methacrylate-co-butyl acrylate) copolymer films. Opt Mater (Amst) 36:804–808. https://doi.org/10.1016/j.optmat.2013.11.030
Vyazovkin S (2010) International Reviews in Physical Chemistry Kinetic concepts of thermally stimulated reactions in solids : A view from a historical perspective. 45–60. https://doi.org/10.1080/014423500229855
Dollimore D, Tong P, Alexander KS (1996) The kinetic interpretation of the decomposition of calcium carbonate by use of relationships other than the Arrhenius equation. Thermochim Acta 282–283:13–27. https://doi.org/10.1016/0040-6031(95)02810-2
Rosu C, Manaila-Maximean D, Paraskos AJ (2002) Thermally stimulated depolarization currents and optical transmission studies on a 3,4-dicyanothiophene-based bent-rod liquid crystal. Mod Phys Lett B 16:473–483. https://doi.org/10.1142/S021798490200397X
Ganea CP, Manaila-Maximean D (2011) Liquid crystal/copolymer-clay nanostructured systems: contribution to the conductivity of the electrode polarization. UPB Sci Bull Series A. 73:209–216. https://www.scientificbulletin.upb.ro/rev_docs_arhiva/full68673.pdf
Pielichowski K, Njuguna J (2005) Thermal Degradation of Polymeric Materials. iSmithers Rapra Publishing, Shrewsbury, Shropshire, UK
Hu YH, Chen CY, Wang CC (2004) Thermal degradation kinetics of poly(n-butyl acrylate) initiated by lactams and thiols. Polym Degrad Stab 84:505–514. https://doi.org/10.1016/j.polymdegradstab.2004.01.009
Doğan F, Kaya İ, Yürekli M (2007) Kinetic of thermal degradation of poly(isobornyl methacrylate). Catal Letters 114:49–54. https://doi.org/10.1007/s10562-007-9041-9
Acknowledgements
This work has been partially accomplished in the framework of an international research program (PHC Tassili). The authors gratefully acknowledge the support of the Algerian Ministry of Higher Education and Scientific Research (MESRS), the General Directorate of Scientific Research and Technological Development (DGRSDT) of Algeria, CampusFrance, the University of Tlemcen/Algeria, the CNRS, and the University and the CROUS of Lille/France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Appendix A
Appendix A
Table: Pyrolysis-GC/MS parameters used to perform pyrolysis experiments.
GC parameters | |
|---|---|
Oven temperature | 40 °C |
Colum flow | 1.71 mL/min |
Injector temperature | 300 °C |
Split ratio | 1/100 |
Gas carrier | He |
GC oven temperature program | (1) hold 95 min at 40 °C (2) 40 °C to 320 °C at 10 °C/min (3) hold 17 min at 320 °C |
MS parameters | |
|---|---|
Source temperature | 230 °C |
Source type | Electron-Impact (EI) ionization source |
m/z scan range | 10—800 |
Software | |
GCMS real time analysis | |
F search (Frontier Lab) | |
Rights and permissions
About this article
Cite this article
Merah, D., Bedjaoui, L., Zeggai, N. et al. Enhanced thermal stability of biobased crosslinked poly (isobornylacrylate-co-2-ethylhexylacrylate) copolymers. J Polym Res 29, 279 (2022). https://doi.org/10.1007/s10965-022-03139-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03139-7