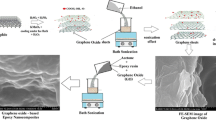
Abstract
Epoxy resins are important industrial polymers due to their versatile properties and ease of preparation. They are used on a large scale in coating and adhesives applications. Epoxy resins are prepared by photo-curing technique or thermal curing method in presence of different hardeners. The first method is used for small areas while the latter is utilized mainly for coating applications. Graphene and its oxide have proved themselves as good fillers for epoxy resin since the formed composites demonstrated enhanced mechanical, thermal and electrical properties. This article gives insight view for synthesis techniques and properties of these composites with a deep discussion for electrical and dielectric investigations of epoxy resins loaded with threshold concentration of graphene oxide. A case study of dielectric properties of epoxy resin/modified graphene oxide composite, is presented in which effect of curing technique on dielectric behavior is explored. Although the amount of filler was kept at the threshold concentration (1% by weight) obvious change in the dielectric properties could be observed unlike the rest of properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epoxy resins are group of polymers that used almost in our daily life. They were firstly synthesized in 1891 but commercially marketed in the 1940’s [1]. The term epoxy is used for pre-polymers and monomers that contains epoxy group within the molecule or as terminal group. Moreover, the cured polymers are called epoxy resin although nearly all the reactive epoxy groups have reacted. The epoxy group is a planner one while after curing a three-dimensional network is formed. Their geometry and chemistry were reviewed in many interesting reviews [2,3,4,5,6]. The different industrial applications relied on these materials needs formation of a network through the reaction of the resin with a suitable curing agent which is known as hardener. The reactivity of the epoxy resin determines the type of the used poly-functional hardener. Aliphatic amines can be used as curing agents at room temperature while acid anhydrides are used for thermal curing. Epoxy resins are used as intermediates in UV-coatings and additives to some coatings to enhance adhesion. Also, they react with fatty acids to yield epoxy polyesters suitable for air-dried coatings. B. Ellis in his book “Chemistry and Technology of Epoxy Resins” gathered valuable information about chemistry, synthesis, properties and applications of epoxy resins [7]. Epoxy resins are characterized by their chemical resistance, good mechanical properties, electrical and thermal insulation and durability which make them the first choice for engineers.
Polymer composites combine the properties of the hosting polymer such as ease of preparation and low cost [8] beside the characteristic features of the added inorganic material such as high aspect ratio and strength. Moreover, the obtained composite may show new properties such as electrical or thermal conductivity [9]. In the last few decades, relying on polymer composites to became essential to obtain materials of enhanced mechanical, thermal or flame resistance properties [10,11,12]. Nevertheless, preventing agglomeration of nanofillers is a challenging task since inhomogeneous dispersion of the nanofiller reduces the mechanical properties of the formed composite. Compositing of epoxy resins, (ERs), with inorganic nanofillers has been extensively reviewed and studied [13,14,15,16]. Lately, interest in investigation of graphene/epoxy resins nanocomposites increased exploring thermal, mechanical and anti-flammable features of the formed nanocomposites. An interesting review has been published concerned with epoxy resin/graphene nanocomposites [17]. The authors highlighted different points among them new methods for the nanocomposites preparation, synergic effect of graphene and other fillers in the epoxy matrix beside the reason behind reduction of thermal stability after incorporation of graphene in the epoxy matrix. Epoxy resin/Graphene nanocomposites were prepared to improve different properties such as electrical, mechanical, rheological, thermal properties among others [18,19,20,21,22,23,24,25,26]. Moreover, studies related to functionalization of graphene for better dispersion and comparison with other nano-carbon materials such as carbon nanotubes were also demonstrated [27,28,29]. The results depicted that the targeted improved properties are better in case of incorporation of graphene in the epoxy matrix than in case of carbon nanotubes.
Graphene (G) is one of carbon’s allotropes that attracted researcher interest in the last two decades due to its unique and versatile properties. It has 2D mono-layer carbon atoms of sp2 structure with benzene rings repeating units [30]. Graphene is characterized by large surface area, superior mechanical properties since it is 200 times harder than steel [31]. It has high thermal stability and exceptional electronic conductivity. The first definition of single sheet of graphite as graphene has been done at 1986 by Boehm [32]. These outstanding properties render it the ideal candidate to many applications such as gas detection [33], nanocomposites [34], energy storage [35], high barrier films [36], among other applications. However, (G) is relatively unstable due to the delocalization of π electrons, so that its sheets aggregate and folds to increase entropy and stability and usually is found in the graphite form [37]. However, graphene oxide (GO), a graphene derivative is more stable and easier in the synthesis from graphite. It is characterized by presence of oxygenated functional group on the sheet surface and on the periphery which enable its modification. GO is a macromolecule with variable properties according to extent of oxidation [38]. In 2004, Novoselov et al. published an article describes the synthesis of (G) in one or few layers by micromechanical technique [39]. The cleavage of graphite has been done by scotch tape and the method is used successively to obtain thinner layers or even one-layer of graphene. The breakthrough of the researchers is the ability to demonstrate the unique electronic structure of graphene. The obtained few layers of graphene can be pressed against another substrate such as SiO2 on silicon to build a device or for further investigation. The great success and simplicity of the micromechanical method encouraged other working groups to investigate possibility of using graphene in building electronic devices. However, the growing interest and demand in graphene and the fact that there is no chance for scale up on using micromechanical method, led to seeking for other production techniques. Graphene fabrication methods can be classified into: bottom-up method such as chemical vapor deposition (CVD) [40], and top-down technique by exfoliating graphite [41]. In the CVD method, graphene is formed in an evacuated chamber under high temperature onto surface of a substrate exposed to a volatile precursor that reacts or decomposes [42, 43]. Silicon or a transition metal is used as substrate and vapors of a hydrocarbon decompose in presence of a catalyst. Graphene layers are formed due to deposition of carbon atoms onto the surface of the substrate. The main advantage of this method is that highly pure, defect free single crystal graphene can be obtained. The good quality of (G) is sufficient for electronic and optical applications. However, transferring (G) sheet without damage from the surface of the substrate to another surface is a challenging task beside the low yield of the formed (G) are the main drawbacks of the method [44]. Additionally, liquid exfoliation of graphite represents a more practical method to obtain amount of G sufficient to be used as filler in polymer composites [45]. In this strategy, graphite is sonicated in presence of a solvent such as N-methylpyrrolidone and a surfactant then centrifuged to remove excess graphite [46, 47]. The graphene suspension showed a noticeable stability over periods of weeks. Exfoliation of graphite in low boiling point solvents such as chloroform and isopropanol has been described [48]. Modification of the method has been described in which water is used instead of the organic solvent and sodium dodeclbenzene sulphonate (SDS) was the dispersing agent [49]. The formed G/SDS was annealed to remove the surfactant and defect-free G could be obtained in good yield. The most common and practical method for graphene synthesis is chemical exfoliation of graphite, (Hammer method) [9]. It is a two-steps method in which graphite is oxidized under harsh conditions to obtain graphite oxide which is then reduced using a powerful reducing agent such as hydrazine to G [50]. This method maintains the flat structure of G and its electrical conductivity but it is still contains lattice defects and considerable amount of oxygenated functionalities. There are other methods for G preparation such as template route method [51], arc-discharge method [52], and total organic synthesis of grapheme [53]. Generally, mechanical exfoliation, CVD and chemical methods are the most common methods for G preparation.
Epoxy resins (ER) are engineering materials characterized by their low cost and ease of processing. They have the peculiarity of low shrinkage upon curing, good adhering to several substrates, high electrical insulation and high chemicals and corrosion resistance. However, in terms of structural applications, they are brittle and temperature sensitive materials. There are several types of epoxy resins. Bisphenol A is a type of ER which is prepared through reaction of epichlorohydrin with bisphenol-A in presence of a basic catalyst. The low molecular weight compound is liquid with low viscosity while the high molecular weight Bisphenol A is solid [54]. Cycloaliphatic epoxy resin, another type, is prepared peracetic acid with cyclohexenylmethyl, and cyclohexene carboxylate. Diglycidyl ester of hexahydrophthalic acid and 3,4-Epoxycyclohexylmethyl-3',4'-epoxycyclohexane, belong to this type of ER. They are used for application require high temperature or UV–resistance. Tri-functional epoxy resin can be produced through reaction of epichlorohydrin and trimethylolpropane while tetrafunctional resin can be obtained by reacting epichlorohydrin with diaminobenzene [55]. Thermosetting resins are prepared through reaction of aromatic novolac and epichlorohydrin and are characterized by high thermal stability and corrosion resistance. Hardening of the resin can be formed thermally or photochemically [56]. There are several types of hardening agents such as amine, anhydrides and alkali-type hardening agent. In case of anhydrides, basic compounds such as tertiary amine catalysts are added. In-photo-curing, the resin can be hardened using UV light, infrared or electron beam irradiation in presence of photoinitiator [57]. UV radical curing is mostly used for coatings, varnishes and paints since the material cures within few seconds. The process is of ecological advantage since no solvent is used and no energy consumption. However, UV cationic curing is recognized due to high adhesion of substrate with low shrinkage. Cycloaliphatic epoxy resins comprise most of the commercial formulations cured using cationic photoinitiators such as aryl sulphonium or iodonium salts [58].
This article is concerned with highlighting different properties of thermally cured in comparison with UV cured epoxy resins filled with graphene or its oxide. Surprisingly, not many studies dealt with dielectric behavior of epoxy resin/G composites which encouraged us to focus on this property in the current work. Dielectric spectroscopy covering a frequency range (10–1 – 107 Hz), was employed to investigate the electrical and dielectric properties at different temperatures ranging from -5 to 150 °C. The raised question here if the curing method affects the electrical and dielectric properties of the epoxy resin and its composites.
Experimental part
Materials
3,4-epoxy cyclohexane carboxylate (ER) and, 3-aminopropyl triethoxysilane (APTES) and N-methyl pyridine were products of Sigma Aldrich. Tetrahydrofurane, chloroform and, 1,2,3,6- tetrahydrophethalic anhydride (THPA) are obtained from fluka. All chemicals were used as received (Scheme 1).
Methods
Synthesis of thermally cured films
A definite weight of ER is thoroughly mixed with the pre-melted 1,2,3,6-tetrahydrophethalic anhydride. The amount of curing agent is added after calculation of the required active anhydride to react with 100 part of epoxide groups. 0.5 mL of N-methyl pyridine is added and the whole mixture was stirred well by magnetic stirrer. The mixture is poured in a Teflon mold and thermally cured in an oven at 120, 150, and 180 °C for 1, 2, and 2 h respectively.
Synthesis of graphene oxide (GO)
Preparation of GO has been performed using well-known Hummer method while the modified form GONSi was prepared by the method described before [26] and can be summarized as follows: 0.8 g of GO dispersed in adequate amount of toluene through sonication then stirring for 2 days. 1.7 mL of APTES mixed with 10 mL chloroform, were added to GO dispersion and stirred for 48 h at 60 °C. After reaction completion, the solvents were removed using rotary evaporator. The modified GO was washed several times using deionized water, separated by centrifugation and finally dried in an oven under vacuum.
Synthesis of thermally cured ER loaded with GO or modified GO
Epoxy resin (15 g) is loaded with different ratios of the filler and dispersed using tip sonicator at amplitude 55%, 0.5 cycles/sec for 30 min. Melted THPA (12 g) and o.5 mL of the catalyst, are added and the mixture was stirred with magnetic stirrer for a few minutes. Finally, the mixture was poured in a Teflon mold. The sample is cured thermally using the previously mentioned heating regime.
Characterization of modified resin
Chemical structure of thermally cured samples was studied using attenuated total reflection (ATR) Fourier transform infrared (FTIR). Spectra were collected with FT-IR Mikroskop Hyperion 2000 coupled with Vertex 70. The range of measurements was 600–4000 cm−1. One hundred scans per spectrum were collected at a resolution of 4 cm-1. Thermal stability of the coatings was studied using a TGA Q 5000 of TA Instruments under nitrogen atmosphere with heating rate 10 °C/min and temperature rang 10° to 800 °C. Differential scanning calorimetry (DSC) analysis was carried out using DSC 2500 of TA-Instruments from Perkin-Elmer. Samples analyzed under N2 atmosphere and heating rate 10 K/min in the temperature range -10° to 350 °C and glass transition temperature (Tg) was evaluated from the second heating cycle. The dielectric spectroscopy measurements were carried out using a high-resolution α-analyzer from NOVOCONTROL Technologies GmbH & Co. KG in temperatures ranging from -50 up to 150 °C and frequency window of 10–1–107 Hz combined with a Quatro temperature controller ensuring absolute thermal stability better than ± 0.5 °C. The sample cells for these measurements consist of two brass electrodes. The lower one is a ground plate of diameter 40 mm. while the upper electrode is of diameter 20 mm. the sample is sandwiched between them in the parallel plate geometry.
Results and discussion
Composites of epoxy resin with different fillers have been prepared to enhance, mechanical, thermal or electrical properties of the resin [59,60,61]. Nanocomposites of epoxy resins and carbon nanotubes (CNT) have been also investigated as toughening and strengthening modifier [62, 63]. Also, CNT prepared by sol–gel method, has been incorporated in an epoxy matrix as flame retardant and for enhancing thermal stability [64]. Nevertheless, due to the high manufacturing cost and anisotropy functionality of CNT, the researchers moved to another 2D material which is graphene nanoplatletes [65]. Because of its low cost, natural abundance, high specific area and higher level of transferring stress across interface, G has gained lot of attention as filler for epoxy resins [66]. The most critical point, is obtaining good dispersion of G in the epoxy resin in order to ensure maximum availability of the surface area of the filler [67]. Most of the reported articles described inclusion of GO in the epoxy matrix due to presence of oxygen functionality which increases its dispersion and reduce filler’s aggregations. There are several methods to obtain GO/epoxy nanocomposite, among them resin impregnation that was described by Im et al. [68] In this method 60% composite has been achieved by dropping epoxy resin containing curing agent onto filter cake of GO previously suspended and dried from water. This method enabled fabrication of highly concentrated nanocomposite with appropriate mechanical properties. In another work, Rehim and Turky prepared UV cured modified GO/ epoxy resin nanocomposite [26]. Modification of GO has been performed by silane moieties to increase dispersion of GO in the resin. Cationic photocuring polymerization of aliphatic epoxy resin was carried out in presence of 0.5 or 1% of functionalized GO. Although, the presence of the filler decreased polymerization process but the formed nanocomposite showed enhanced thermal stability. In case of thermally cured composites, successful modification of GO with amino silane moieties is confirmed by increasing the aliphatic CH2 vibration band at 2980 cm−1. A new band appeared at 1090 cm−1 related to Si–O-Si and a band at 800 cm−1 corresponding to Si–O-C in the spectrum of ER GONSi [69]. A band related to carbonyl group can be noticed at 1722 cm−1 (shown in Fig. 1). Functionalization of GO with amino silane coupling agent then incorporation of 0.2% of the filler in epoxy resin yielded 32% increase in Young’s modulus (3.3 GPa) and 16% increase in tensile strength (81.2 MPa) of the formed nanocomposite [70]. Mechanical blending of GO with epoxy resin via a Haake MinilabII, mini-lab twin screw blender is described [71]. The addition of small ratios of GO in the thermally conductive epoxy matrix offered fire retardancy as confirmed by oxygen limiting technique and other testing methods. Huskić et al. studied the effect of GO particle size on the formed GO/ epoxy resin [72]. The formed nanocomposite showed increased Young’s modulus by 35% for GO of 130 μm particle size and 30% or 1200 μm particle size while tensile strength increased by only 10% for GO of 1200 μm. Investigation of glass transition temperature of epoxy resin (ER) and loaded samples was carried out using DSC analysis. (Fig. 2) It was noticed that pure ER has Tg value of 165 °C while on addition of 0.5% of GONSi a pronounced reduction in the value of Tg was measured (120 °C) mostly because of plasticizing effect of modified filler. Surprisingly, addition of double of the filler amount to ER (1wt% GONSi) led to small decrease in Tg value to be 115 °C. Lately, a review has been published demonstrating improvement of thermal and mechanical properties of composites based on nanocarboneous materials [73]. The authors concluded that the best mechanical and thermal properties for the prepared composites can be attained via good dispersability of the filler in the epoxy matrix. Moreover, functionalization of GO with modifiers containing amine groups is significantly important since the amine groups can stabilize the dispersion of GO in the polymer matrix.
Electrical and dielectric study
Electrical properties of epoxy resins reinforced with graphene’s nanosheets or its derivative GO is investigated thoroughly. The broadband dielectric spectroscopy is employed to investigate the complex dielectrics function ε∗ (ω, T) = ε'(ω, T) ‒ ε''(ω, T), where ε' is the permittivity and ε'' is the dielectric loss. It is equivalent with the complex conductivity function σ∗ (ω, T) = σ'(ω, T) + σ''(ω, T) since, σ∗ (ω, T) = iωεoε∗ (ω, T), implying that σ' = εoωε'' and σ'' = εoωε' (εo being the vacuum permittivity). However, as G is a conductive material, the electrical conductivity of the composites varies with the method of their preparation. Imran et al. prepared G/epoxy resin composite using three different techniques, namely, mechanical mixing, sonication and a three roll mill dispersion method. The electrical conductivity values for samples contain 1 wt % of G, were 2 × 10–12, 1 × 10–7 and 2 × 10–6 S/cm, respectively [74]. The composite prepared by three-roll mill processing technique depicted the best electrical conductivity value owing to the higher dispersion of the filler in the epoxy matrix. A high dielectric constant material is prepared by loading a bisphenol resin with graphite nano-platelets. The thermally cured composites filled with 0.949 vol.% depicted high dielectric constant and low loss tangent at around 104 Hz [75]. It is believed that the increase in dielectric constant is a result of the interfacial polarization and the mini-capacitors model [76]. Investigation of dielectric properties of the ternary system RGO-Fe2O3/epoxy resin demonstrated decrease in the dielectric constant at low frequency value then remains constant [77]. Additionally, increase in the DC conductivity with the filler content led to increase in ε″ of the composites, increase in interfacial polarization and increase in crystallinity of the samples. The enhancement in DC conductivity can be attributed to the presence of RGO-Fe2O3 which introduces charge carriers that facilitate mobility of charges. Rahnamol et al. has developed a hybrid composite of polyaniline and RGO to obtain a high dielectric material and added it to an epoxy resin [78]. The results showed that the electrical conductivity of the composite containing 9 wt.% of the filler increased five folds in addition to that an enhancement of dielectric constant was achieved. It is worth to mention that little is mentioned in the literature about the dielectric behavior of composites at composition threshold. In particular applications such as cable joints and terminations, it is required to modulate conductivity of electrical insulation over a few decades of values to avoid field concentration [79]. In this context, Toselli et al. investigated in a wide range of frequencies, the dielectric properties (permittivity and loss factor) of epoxy resin loaded with 0.5% GO as well as its electrical conductivity [80]. The influence of the filler has been investigated in 4 different temperatures starting from -20 up to 60 °C. The results demonstrated that by increasing temperature the imaginary permittivity peak relates to mobility of small segments, this peak moves toward higher frequency while the real part of permittivity increases with due to dipole mobility. Additionally, post-curing heat treatment led to slight permittivity decrease due to possible cross linking and reduction of free volume and reduction in chain mobility [81]. In another study, dielectric properties of UV-cured epoxy resin filled with small amounts of modified GO, is investigated over broad range of temperatures (-40 °C -160 °C) [26]. Two trends of increasing ε″ with temperatures could be observed with increase in the rate of change after the calorimetric glass transition temperature Tg. While at low temperature, a relaxation peak can be observed due to dipolar orientation of hydroxyl end groups. The absence of any relaxation peak of the space charge effect (α-polarization) could be attributed to the higher degree of homogeneous structure of the composite due to lower concentration of the filler (0.5 and 1 wt. %). In the case of thermally cured epoxy resin modified with small amounts of aminosilane-functionalized graphene oxide (GO NSi) prepared in this study and studied using the same technique (BDS) over a wide range of frequency and temperatures. It was found that the temperature dependence of the imaginary part of permittivity, ε″ (usually called dielectric loss) shows similar behavior, not shown here, as that noticed previously in the UV-cured epoxy resin filled with the same concentration of modified GO [26]. To better understand the effect of curing system, on the dielectric and/or electrical properties of the investigated composites we will present ε″ against frequency at lower temperatures for deep insight of the molecular dynamic relaxation peak. Before we go through this issue, let us consider the real part of the complex permittivity,ε′, that usually characterizes the different polarizations take place at the molecular scale. The real part of the complex permittivity, ε', was measured at fixed frequency point 1 kHz as a representative example for the three samples (that is, unloaded aliphatic epoxy resin, ER, and ER loaded with modified GO [ER GONSi] @ concentrations 0.5 and 1%) and has been illustrated graphically against temperatures in Fig. 3.
The figure shows a gradual and very slight increase in (ε′), with increasing temperature till about 70 °C. Further increase of temperature shows different trends of ER-free and both ER samples loaded with modified GO [ER GONSi]. However, the ER-free shows remarkably increase of the rate of change due to the expected increase of fluctuations at the molecular scale; there is clear restriction of the dynamics due to the filler added in other both samples. In order to characterize the molecular dynamics of the investigated samples, the dielectric loss is presented versus frequency at three lower temperatures, namely, -50, -40 and -30 °C [Fig. 4].
The figure depicts clearly two dynamic peaks for samples under investigations, the high frequency one is developed at about 1 kHz and shifted towards higher frequencies as temperature increases. It is the so called β-relaxation process attributed to the fluctuations of the functional hydroxyl groups. This accompanied by a gradual increase in the real permittivity according to the enhanced dipoles’ mobility. This dynamic was discussed in some details in case of photo synthesis of similar samples [26]. However, there is also some other opinions according to the origin of this dynamic [82,83,84,85]. No conductivity contribution to the dielectric representation could be seen here. One has to take in mind the insulation features of the samples and the lower temperatures of measurements which reduced the charge transport. The other relaxation peak shown at lower frequencies (slower dynamic) is also secondary dynamic β′-relaxation process. This is due to the fluctuation of the ethoxy groups (OCH2CH3) confirming what was found in ATR-FTIR spectra. There is in general gradual increase in the dielectric loss, ε″, values with the GONSi loadings. This is of course reflects the effect of heterogeneous structure of the investigated complexes causes interfacial polarization usually found in polymer nanocomposites [86,87,88,89,90,91] or what is attributed to the so-called micro-capacitors effect [92, 93]. The frequency dependence of the real conductivity, σ′, for the three samples under consideration is shown in Fig. 5 at two different temperatures namely 100 and -40 C.
The figure shows gradual decrease, almost linear, of σ′ with decreasing frequency for the three samples at the lower temperature without indication of the dc plateau. This is of course a main feature of the perfect insulation character as discussed previously [85, 93]. The values of conductivity at 100 mHz are in the range of FS/cm [i.e. 10–15 Scm−1] and slightly affected by GONSi loading as follows: ER, GONSi 05 and GONSi 1 have conductivity 1, 2.4 and 4.08 FS/cm. Increasing the measuring temperature to be 100 C increased these values to become: 190, 19,000 and 53,000 FS/cm. However, it is still in all cases insulators. The ac-conductivity tends to be plateaued at lower frequencies for the two samples GONSi 05 and GONSi 1 representing the dc-conductivity.
Conclusion
Synthesis and properties of epoxy resins loaded with graphene is demonstrated in this work. Epoxy resins (ER) filled with concentrations of GO up to 1wt% is deeply discussed since the properties of the formed material do not change while electric and dielectric properties are varied significantly. Deep study of dielectric properties of ER loaded with modified GO showed absence of any relaxation peak of the space charge effect (α-polarization). This dynamic peak relaxation became now a main feature of the dielectric relaxation in the heterogeneous structure nanocomposites. This reflects, without doubt the effect of charge carriers’ transport which screened out any effect of the interfacial polarizations. A very slight increase in (ε′), with elevation of temperature till about 70 °C could be noticed for both pure and modified resin. Further increase of temperature shows remarkable increase in the rate of change in the ER sample due to the expected increase of fluctuations at the molecular scale unlike the loaded samples that suffer from restriction of the fluctuation. The dielectric loss spectra, ε″(ν), represented at relatively lower temperatures show micro-capacitors effect due to the heterogeneous structure of the investigated complexes that causes interfacial polarization. Even, the ac-conductivity shows a perfect insulation behavior of the all samples at lower temperatures—ac-conductivity @ 0.1 Hz is only some femto Siemens per cm at -40 °C, they became more conductive at higher temperatures in comparison with those samples prepared using photo-cured technique. In this case the behavior of the ac-conductivity follows the well-known Jonscher model. According to the fact that dc-conductivity is just the multiplication of the density and mobility of charge carriers, the development of the dc-conductivity plateau in this case may reflect a development of free charge carriers accompanied by free paths.
References
Dearborn EC, Fuoss RM, Mackenzie AK, Shepherd RG (1953) Epoxy resins from bis-, tris-, and tetrakis-glycidyl ethers. Ind Eng Chem 45:2715
Parker RE, Isaacs NS (1959) Mechanisms of epoxide reactions. Chern Rev 59:737–799
Lewars EG (1984) Comprehensive heterocyclic chemistry, ed. Katritsky, A.R and Rees, C.W. Vol. 7, section 5.0.5.4, ed. Lwowski, W. Pergamon Press, Oxford, pp. 114–118
Katritsky AR, Weeds SM (1966) Adv Heterocycl Chem 7(225–299):232–233
Katritsky AR, Jones PM (1979) Adv Heterocycl Chem 25(303–391):327–329
Belen'kii LI (1988) Adv. in Heterocyclic Chemistry 44,269–396. Synthesis of epoxy groups p. 304. Reactions of epoxy groups, pp. 303–330.
Ellis B (1993) Chemistry and technology of epoxy resins. Springer Science+ Business Media Dordrecht
Deng S, Djukic L, Paton R, Ye L (2015) Compos A 68:121–132
Mittal G, Dhand V, Rhee KY, Park SJ, Lee WR (2015) A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J Ind Eng Chem 21:11
Heo Y, Im H, Kim J, Kim J (2012) The influence of Al(OH)3-coated graphene oxide on improved thermal conductivity and maintained electrical resistivity of Al2O3/epoxy composites. J Nanopart Res 14:1–10
Wang X, Xing W, Feng X, Yu B, Lu H, Song L, Hu Y (2014) The effect of metal oxide decorated graphene hybrids on the improved thermal stability and the reduced smoke toxicity in epoxy resins. J Chem Eng 250:214–221
An JE, Jeong YG (2013) Structure and electric heating performance of graphene/epoxy composite films. J Eur Polym 49(6):1322–1330
Becker O, Simon GP (2005) Epoxy layered silicate nanocomposites. Adv Polym Sci 170:29–82
Karak N (2006) Polymer (epoxy) clay nanocomposites. J Polym Mater 23:1–20
Sua L, Zengb X, Hec H, Taocd Qi, Komarneni S (2017) Preparation of functionalized kaolinite/epoxy resin nanocomposites with enhanced thermal properties. Appl Clay Sci 148:103–108
Cortés P, Fraga I, Calventus Y, Román F, Hutchinson JM, Ferrando F (2014) A new epoxy-based layered silicate nanocomposite using a hyperbranched polymer: Study of the curing reaction and nanostructure development. Materials 7:1830–1849
Wei J, Vo T, Inam F (2015) Epoxy/graphene nanocomposites – processing and properties: a review. RSC Adv 5:73510
Sanjinés R, Abad MD, Vâju C, Smajda R, Mioni´c M, Magrez A (2011) Electrical properties and applications of carbon based nanocomposite materials: An overview. Surf Coat Technol 206:727–733
Al-Saleh MH, Sundararaj U (2011) Review of the mechanical properties of carbon nanofiber/polymer composites. Compos Part A 42:2126–2142
Shahil KMF, Balandin AA (2012) Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun 152:1331–1340
Potts JR, Dreyer DR, Bielawski CW, Ruoff RS (2011) Graphene-based polymer nanocomposites. Polymer 52:5–25
Qin F, Brosseau C (2012) A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles. J Appl Phys 111:061301. https://doi.org/10.1063/1.3688435
Lee S-Y, Park S-J (2012) Comprehensive review on synthesis and adsorption behaviors of graphene-based materials. Carbon Lett 13:73–87
Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S (2011) Graphene-based materials: past, present and future. Prog Mater Sci 56:1178–1271
Van Rooyen LJ, Karger-Kocsis JJ, Kock LD, David Kock L (2015) Improving the helium gas barrier properties of epoxy coatings through the incorporation of graphene nanoplatelets and the influence of preparation techniques. J Appl Polym Sci 42584:1–13
Rehim MA, Turky G (2019) Silane-functionalized graphene oxide/epoxy Resin nanocomposites: Dielectric and thermal studies. J Appl Polym Sci 136(47):48253
Naeema M, Kuan HC, Michelmore A, Meng Q, Qiu A, Aakyiir M, Losic D, Zhu S, Ma J (2020) A new method for preparation of functionalized graphene and its epoxy nanocomposites. Compos Part B Eng 196:108096
Liu T, Zhao Z, Tjiu WW, Lv J, Wei C (2014) Preparation and characterization of epoxy nanocomposites containing surface-modified graphene oxide. J Appl Polym Sci 40236–40242
Zhang J, Zheng Y, Zhou H, Zhang J, Zou J (2012) The Influence of Hydroxylated Carbon Nanotubes on Epoxy Resin Composites. Adv Mater Sci Eng 2012(518392):5
Geim AK (2009) Graphene: Status and prospects. Science 324:1530–1534
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Boehm H, Setton R, Stumpp E (1986) Nomenclature and terminology of graphite intercalation compounds. Carbon 24:241–245
Zhang H, Lee HK (2011) Plunger-in-needle solid-phase microextraction with graphene-based sol-gel coating as sorbent for determination of polybrominated diphenyl ethers. J Chromatogr A 1218:4509–4516
Young RJ, Kinloch IA, Gong L, Novoselov KS (2012) The mechanics of graphene nanocomposites: A review. Compos Sci Technol 72:1459–1476
Sanjinés R, Abad MD, Vâju C, Smajda R, Mionic M, Magrez A (2011) Electrical properties and applications of carbon based nanocomposite materials: An overview. Surf Coatings Technol 206:727–733
Yoo BM, Shin HJ, Yoon HW, Park HB (2014) Graphene and graphene oxide and their uses in barrier polymers. J Appl Polym Sci 131(39628):1–23
Keyte J, Pancholi K, Njuguna J (2019) Recent developments in graphene oxide/epoxy carbon fiber-reinforced composites. Front Mater 6:224
Feicht P, Siegel R, Thurn H, Neubauer JW, Seuss M, Szabó T et al (2017) Systematic evaluation of different types of graphene oxide in respect to variations in their in-plane modulus. Carbon 114:700–705. https://doi.org/10.1016/j.carbon.2016.12.065
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV et al (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669
Na SR, Suk JW, Tao L, Akinwande D, Ruoff RS, Huang R, Liechti KM (2015) Selective mechanical transfer of graphene from seed copper foil using rate effects. ACS Nano 9:1325–1335
Soldano C, Mahmood A, Dujardin E (2010) Production, properties and potential of graphene. Carbon 48:2127–2150
Li X, Cai W, An J, Kim S, Nah J, Yang D, Ruoff RS (2009) Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324:1312–1314
Kim KS, Zhao Y, Jang H, Lee SY, Hong BH (2009) Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nat Mater 457:706–710
Whitener KE Jr, Sheehan PE (2014) Graphene synthesis. Diam Relat Mater 46:25–34
Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun Z, De S et al (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 3:563–568
Lotya M, Hernandez Y, King PJ, Smith RJ, Nicolosi V, Karlsson LS et al (2009) Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J Am Chem Soc 131(10):3611–3620
Lotya M, King PJ, Khan U, De S, Coleman JN (2010) High-concentration, surfactant stabilized graphene dispersions. ACS Nano 4(6):3155–3162
O’Neill A, Khan U, Nirmalraj PN, Boland J, Coleman JN (2011) Graphene dispersion and exfoliation in low boiling point solvents. J Phys Chem C 115(13):5422–5428
Ghanem AF, Abdel Rehim MH (2018) Assisted tip sonication approach for graphene synthesis in aqueous dispersion. Biomedicines 6(2):63
Pei S, Cheng H (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Zhang W, Cui J, Tao CA, Wu Y, Li Z, Ma L, Wen Y, Li G (2009) a strategy for producing pure single-layer graphene sheets based on a confined self-assembly approach. Angew Chem Int Ed 121:5978–5982
Wu Z, Ren W, Gao L, Zhao J, Chen Z, Liu B, Tang D, Yu B, Jiang C, Cheng H (2009) Synthesis of graphene sheets with high electrical conductivity and good thermal stability by hydrogen arc discharge exfoliation. ACS Nano 2:411–417
Sakamoto J, Heijst J, Lukin O, Schlüter AD, (2009) Angew. Chem., Two-dimensional polymers: Just a dream of synthetic chemists? Int Ed 48:1030–1069
Jin FL, Ma CJ, Park SJ (2011) Thermal and mechanical interfacial properties of epoxy composites based on functionalized carbon nanotubes. Mater Sci Eng 528:8517–8522
Park SJ, Jin FL, Lee JR (2014) Thermal and mechanical properties of tetrafunctional epoxy resin toughened with epoxidized soybean oil. Mater Sci Eng 374:109–114
Bascom W, Cottington R, Jones R, Peyser P (1975) The fracture of epoxy- and elastomer-modified epoxy polymers in bulk and as adhesives. J Appl Polym Sci 19:12545–12562
Foix D, Fernández-Francos X, Ramis X, Serra A, Sangermano M (2011) New pegylated hyperbranched polyester as chemical modifier of epoxy resins in UV cationic photocuring. Reac Funct Polym 71:417
Kwak GH, Park SJ, Lee JR (2000) Thermal stability and mechanical behavior of cycloaliphatic–DGEBA epoxy blend system initiated by cationic latent catalyst. J Appl Polym Sci 78:290–297
Park SJ, Jin FL, Lee JR (2004) Thermal and mechanical properties of tetrafunctional epoxy resin toughened with epoxidized soybean oil. Mater Sci Eng 374:109–114
Lee MC, Ho TH, Wang CS (1996) Synthesis of tetrafunctional epoxy resins and their modification with polydimethylsiloxane for electronic application. J Appl Polym Sci 62:217–225
Ferdosian F, Ebrahimi M, Jannesari A (2013) Curing kinetics of solid epoxy/DDM/nanoclay: Isoconversional models versus fitting model. Thermochim Acta 568:67–73
Gojny FH, Wichmann MH, Fiedler B, Schulte K (2005) Influence of different carbon nanotubes on the mechanical properties of epoxy matrix composites–a comparative study. Compos Sci Technol 65:2300–2313
Roy S, Petrova RS, Mitra S (2018) Effect of carbon nanotube (CNT) functionalization in epoxy-CNT composites. Nanotechnol Rev 7(6):475–485
Kuan CF, Chen WJ, Li YL, Chen CH, Kuan HC, Chiang CL (2010) Flame retardance and thermal stability of carbon nanotube epoxy composite prepared from sol–gel method. J Phys Chem Solids 71539–543
Zaman I, Kuan H-C, Dai J, Kawashima N, Michelmore A, Sovi A, Dong S, Lee Luong L, Ma J (2012) From carbon nanotubes and silicate layers to graphene platelets for polymer nanocomposites. Nanoscale 4:4578–4586
Singh NP, Gupta VK, Singh AP (2019) Graphene and carbon nanotube reinforced epoxy nanocomposites: A review. Polymer 180:121724
Zhang Y, Wang Y, Yu J, Chen L, Zhu J, Hu Z (2014) Tuning the interface of graphene platelets/epoxy composites by the covalent grafting of polybenzimidazole. Polymer 55:4990–5000
Im H, Kim J (2012) Thermal conductivity of a graphene oxide–carbon nanotube hybrid/epoxy composite. Carbon 50:5429–5440
Wang Z, Wei P, Qian Y, Liu J (2014) The synthesis of a novel graphene-based inorganic–organic hybrid flame retardant and its application in epoxy resin. Compos Part B 60:341–349
Lia Z, Wang R, Young RJ, Deng L, Yang F, Hao L, Jiao W, Liu W (2013) Control of the functionality of graphene oxide for its application in epoxy nanocomposites. Polymer 54(23):6437–6446
Sabet M, Soleimani H, Hosseini S, Mohammadian E (2021) Impact of graphene oxide on epoxy resin characteristics. High Perform Polym 33(2):165–175
Huskić M, Bolka S, Vesel A, Mozetič M, Anžlovar A, Vizintin A, Žagar E (2018) One-step surface modification of graphene oxide and influence of its particle size on the properties of graphene oxide/epoxy resin nanocomposites. Eur Polym J 101:211–217
Benega MAG, Silva WM, Schnitzler MC, Andrade RJE, Ribeiro H (2021) Improvements in thermal and mechanical properties of composites based on epoxy-carbon nanomaterials - A brief landscape. Polym Test 98:107180
Imran KA, Shivakumar KN (2018) Enhancement of electrical conductivity of epoxy using graphene and determination of their thermo-mechanical properties. J Reinf Plast Compos 37(2):118–133
Min C, Demei Yu, Cao J, Wang G, Feng L (2013) A graphite nanoplatelet/epoxy composite with high dielectric constant and high thermal conductivity. Carbon 55:116–125
Deng Y, Zhang YJ, Xiang Y, Wang GS, Xu HB (2009) Bi2S3-BaTiO3/ PVDF three-phase composites with high dielectric permittivity. J Mater Chem 19:2058–2061
Bindu Sharmila TK, Antony JV, Jayakrishnan MP, Sabura Beegum PM, Thachil ET (2016) Mechanical, thermal and dielectric properties of hybrid composites of epoxy and reduced graphene oxide/iron oxide. Mater Des 90:66
Rahnamol AM, Gopalakrishnan J (2020) Improved dielectric and dynamic mechanical properties of epoxy/polyaniline nanorod/in situ reduced graphene oxide hybrid nanocomposites. Polym Compos 41(8):2998–3013
Burns NM, Eichhorn RM, Reid CG (1992) Stress controlling semiconductive shields in medium voltage power distribution cables. IEEE Electr Insul Magz 8:8
Toselli M, Fabiani D, Mancinelli P, Fréchette M, Heid T, David E, Saccani A (2015) In situ thermal reduction of graphene oxide forming epoxy nanocomposites and their dielectric properties. Polym Compos 36(2):294–301
Jonscher AK (1983) Dielectric relaxation in solids. Chelsea Dielectric Press, London, UK
Arridge RGC, Speake JH (1972) Mechanical relaxation studies of the cure of epoxy resins: 2. Activation energy of the γ-process in amine-cured epoxy resins. Polymer 13:450
Soulintzis A, Kontos G, Karahaliou P, Psarras GC, Georga SN, Krontiras CA (2009) Dielectric Relaxation Processes in Epoxy Resin-ZnO Composites. J Polym Sci Part B: Polym Phys 47:445
Darwish AG, Turky G, Hassaan MY, Ghoneim A (2019) Impact of RGO on electrical and dielectric properties of Co3O4/RGO nanocomposite. Mater Res Express 6:105039
Moussa MA, Rehim MHA, Ghoneim AM, Khairy SA, Soliman MA, Turky GM (2019) Dielectric investigations and charge transport in PS-PAni composites with ionic and nonionic surfactants. J Phys Chem Solids 133:163–170
Kremer F, Schonhals A (2003) Broadband dielectric spectroscopy, Springer, pp 59
Omara SS, Rehim MA, Ghoneim A, Madkour S, Thünemann AF, Turky G, Schönhals A (2015) Structure-property relationships of hyperbranched polymer/kaolinite nanocomposites. Macromol 48:6562
Moussa MA, Ghoneim MA, Hakim AAA, Turky GM (2009) Electrical and thermal properties of nylon6/calcium carbonate composites. Adv Polym Technol 28:257–266
Turky GM, Ghoneim AM, Kyritsis A, Raftopoulos K, Moussa MA (2011) Dielectric dynamics of some nylon 6/CaCO3 composites using broadband dielectric spectroscopy. J Appl Polym Sci 122:2039–2046
Dang ZM, Lin YH, Nan CW (2003) Novel ferroelecric polymer composites with high dielectric constants. Adv Mater 15:1625
Dang ZM, Wang L, Yin Y, Zhang Q, Lei QQ (2007) Giant dielectric permittivities in functionalized carbon-nanotube/electroactive-polymer nanocomposites. Adv Mater 19:852–857
Dang Z-M, Yuan J-K, Zha J-W, Zhou T, Li S-T, Hu G-H (2012) Fundamentals, processes and applications of high-permittivity polymer–matrix composites. Prog Mater Sci 57:660
Ibrahim S, Rehim MA, Turky G (2018) Dielectric study of polystyrene/polycaprolactone composites prepared by miniemulsion polymerization. J Phys Chem Solids 119:56–61. https://doi.org/10.1016/j.jpcs.2018.03.030)
Acknowledgements
The financial support of this work provided by Science and Technology Development Fund (STDF), project no. (25401) through short-term fellowship program is deeply acknowledged. The authors are thankful for Prof. B. Voit-Institute for Polymer Research-Dresden-Germany for the FTIR measurements.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rehim, M.A., Turky, G. Epoxy resin reinforced with graphene derivatives: physical and dielectric properties. J Polym Res 29, 120 (2022). https://doi.org/10.1007/s10965-022-02971-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-02971-1