Abstract
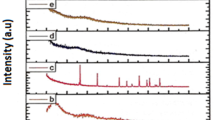
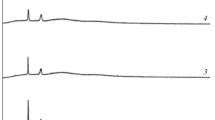
Electrolyte membranes with PEO and PVdF complexed with KNO3 have been prepared using solution cast technique. Succinonitrile has been added in different weight percentages to PEO:PVdF + KNO3 blend polymer electrolyte system. The prepared electrolyte membranes were characterized using X-ray diffraction, Scanning Electron Microscopy, DSC and Fourier Transform IR Spectroscopy to examine the structural properties. Impedance spectroscopic analysis was carried out (between the frequency 101 – 30 × 106 Hz) for wide range (0–15 wt%) of succinonitrile concentrations on blend polymer electrolyte membranes in the temperature range of 303 K-353 K. The ionic conductivity of the electrolyte membrane has been enhanced with succinonitrile concentration and maximum ionic conductivity (8.79 × 10–5 S/cm) was identified for 12wt% at room temperature. The temperature dependent ionic conductivity followed the Arrhenius relation and is attributed to increase in ion dissociation which allows greater number of charge carriers for ion transport in the electrolyte. Wagner’s method has been used to investigate the transference number.












Similar content being viewed by others
References
Richards WD, Wang Y, Miara LJ et al (2016) Design of Li1+2xZn1−xPS4, a new lithium ion conductor. Energy Environ Sci 9:3272–3278
Nechaev GV, Burmakin EI (2011) Potassium-conducting solid electrolytes in K1–2x Srx GaO2 system. Russ J Electrochem 47:1411–1414
Hayashi A, Masuzawa N, Yubuchi S et al (2019) A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature. Nat commun 10:5266
Aziz SB, Abdullah OG, Hussein SA (2018) Role of Silver Salts Lattice Energy on Conductivity Drops in Chitosan Based Solid Electrolyte: Structural, Morphological and Electrical Characteristics. J Elec Materi 47:3800–3808
Reddy YG, Awasthi AM, Chary AS, Reddy SN (2018) Characterization and ion transport studies through impedance spectroscopy on (1–x)Pb(NO3)2:xAl2O3 composite solid electrolytes. Emergent Mater 1:175–184
Raju A, Jeedi VR, Yalla M, Swarnalatha R, Reddy SN, Chary AS (2020) Characterization, ionic conductivity and impedance studies of potassium nitrate. Sol State Technol 63:7824–7835
Hao M, Li J, Park S et al (2018) Efficient thermal management of Li-ion batteries with a passive interfacial thermal regulator based on a shape memory alloy. Nat Energy 3:899–906
Luo JY, Cui WJ, He P et al (2010) Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat Chem 2:760–765
Wang Y, Yi J, Xia Y (2012) Recent progress in aqueous lithium-ion batteries. Adv Energy Mater 2:830–840
Jia W, Fan C, Wang L, Wang Q et al (2016) Extremely Accessible Potassium Nitrate (KNO3) as the High Efficient Electrolyte Additive in Lithium Battery. ACS Appl Mater Interfaces 8:15399–15405
Tong J, Wu S, von Solms N, Liang X, Huo F, Zhou Q, He H, Zhang S (2020) The Effect of Concentration of Lithium Salt on the Structural and Transport Properties of Ionic Liquid-Based Electrolytes. Front Chem 7:945
Sun H, Zhu G, Xu X et al (2019) A safe and non-flammable sodium metal battery based on an ionic liquid electrolyte. Nat Commun 10:3302
Park JW, Ueno K, Tachikawa N, Dokko K, Watanabe M (2013) Ionic liquid electrolytes for lithium-sulfur batteries. J Phys Chem C 117(40):20531–20541
Wright PV (1975) Electrical Conductivity in ionic complexes of poly(ethylene oxide). Br Polym J 7:319–327
Sreekanth T, Reddy MJ, Subramanyam S, Rao US (1999) Ion conducting polymer electrolyte films based on (PEO+KNO3) system and its application as an electrochemical cell. Mater Sci Engg B 64:107–112
Aili D, Jankova K, Han J et al (2016) Understanding ternary poly(potassium benzimidazolide)-based polymer electrolytes. Polym 84:304–310
Jeedi VR, Narsaiah EL, Yalla M, Swarnalatha R, Reddy SN, Chary AS (2020) Structural and electrical studies of PMMA and PVdF based blend polymer electrolyte. SN App Sci 2:2093
Alarco PJ, Abu-Lebdeh Y, Abouimrane A, Armand M (2004) The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat Mater 3:476–481
Gupta RK, Rhee HW (2013) Plasticizing effect of K+ ions and Succinonitrile on Electrical conductivity of [poly(ethylene oxide)-Succinonitrile]/KI-I2 Redox-couple solid polymer electrolyte. J Phys Chem B 117:7465–7471
Mallaiah Y, Jeedi VR, Swarnalatha R, Raju A, Reddy SN, Chary AS (2021) Impact of polymer blending on ionic conduction mechanism and dielectric properties of sodium based PEO-PVdF solid polymer electrolyte systems. J Phys Chem Solids 155:110096
Adams DM, Hatton PD, Heath AE, Russell DR (1988) X-ray diffraction measurements of potassium nitride under high pressure using synchrotron radiation. J Phys C: Solid State Phys 21:505
Takahashi Y, Tadokoro H (1973) Structural studies of polyethers, (-(CH2)m-O-)n. X. Crystal structure of poly(ethylene oxide). Macromolecules 6:672–675
Chu PP, Reddy MJ, Tsai J (2004) Structural and transport characteristics of polyethylene oxide/phenolic resin blend solid polymer electrolytes. J Polym Sci B Polym Phys 42:3866–3875
Bai H, Wang X, Zhou Y, Zhang L (2012) Preparation and characterization of poly(vinylidene fluoride) composite membranes blended with nano-crystalline cellulose. Progress in Nat Sci: Mater Int 22(3):250–257
Yoshihara T, Tadokoro H, Murahashi S (1964) Normal vibrations of the polymer molecules of helical conformation. IV. Polyethylene oxide and polyethylene-d4 Oxide. J Chem Phys 41:2902
Ganta KK, Jeedi VR, Vijaya Kumar K, Laxmi Narsaiah E (2020) Preparation, characterization and impedance spectroscopic studies of Na+ ion conducting PEO + PVDF-blended polymer electrolytes. Int J Polym Anal Charact 26:130–144
Pandey GP, Liu T, Hancock C, Li Y, Sun XS, Li J (2016) Thermostable gel polymer electrolyte based on succinonitrile and ionic liquid for high-performance solid-state supercapacitors. J Power Sources 328:510–519
Chu PP, Reddy MJ (2003) Sm2O3 composite PEO solid polymer electrolyte. J Power Sources 115:288–294
Tang Z, Wang J, Chen Q et al (2007) A novel PEO-based composite polymer electrolyte with absorptive glass mat for Li-ion batteries. Electrochim Acta 52:6638–6643
Ge X, Changjing Fu, Chan SH (2011) Double layer capacitance of anode/solid-electrolyte interfaces. Phys Chem Chem Phys 13:15134–15142
Wu XL, Xin S, Seo HH, Kim J, Guo YG, Lee JS (2011) Enhanced Li+ conductivity in PEO–LiBOB polymer electrolytes by using succinonitrile as a plasticize. Solid State Ionics 186:1–6
Johan MR, Shy OH, Ibrahim S et al (2011) Effects of Al2O3 nanofiller and EC plasticizer on the ionic conductivity enhancement of solid PEO-LiCF3SO3 solid polymer electrolyte. Solid Stat Ionics 196:41–47
Ganta KK, Jeedi VR, Katrapally VK et al (2021) Efect of TiO2 Nano-Filler on Electrical Properties of Na+ Ion Conducting PEO/PVDF Based Blended Polymer Electrolyte. J Inorg Orgomet Polym 31:3430–3440
Ibrahim S, Yasin SMM, Ahmad R et al (2012) Effect of various plasticizer concentrations on salted PEO based solid polymer electrolytes. Int J Plastic Tech 16:125–135
Arya A, Sharma AL (2019) Tailoring of the structural, morphological, electrochemical, and dielectric properties of solid polymer electrolyte. Ionics 25:1617–1632
Das S, Ghosh A (2017) Charge Carrier Relaxation in Different Plasticized PEO/PVDF-HFP Blend Solid Polymer Electrolytes. J Phys Chem 121(21):5422–5432
Wang H, Lin C, Yan X, Wu A, Shen S, Wei G, Zhang J (2020) Mechanical Property-reinforced PEO/PVDF/LiClO4/SN blend all solid polymer electrolyte for lithium ion batteries. J Electroanalyt Chem 869(15):114156
Dhatarwal P, Sengwa RJ (2017) Dielectric and electrical characterization of (PEO–PMMA)–LiBF4–EC plasticized solid polymer electrolyte films. J Polym Res 24:135
Manjuladevi R, Thamilselvan M, Selvasekarapandian S et al (2017) Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)- poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Singh NL, Shah S, Qureshi A, Tripathi A, Singh F, Avasthi DK, Raole PM (2011) Effect of ion beam irradiation on metal particle doped polymer composites. Bull Mater Sci 34:81–88
Ramesh S, Ang GP (2010) Impedance and FTIR studies on plasticized PMMA–LiN (CF3SO2)2 nanocomposite polymer electrolytes. Ionics 16:465–473
Zaki HM (2005) AC conductivity and frequency dependence of the dielectric properties for copper doped magnetite. Phys B Condens Matter 363:232–244
Itoh T, Fujita K, Uno T, Kubo M (2017) Polymer electrolytes based on vinyl ethers with various EO chain length and their polymer electrolytes cross-linked by electron beam irradiation. Ionics 23:257–264
Alo Dutta TP, Sinha P, Jena SA (2008) AC conductivity and dielectric relaxation in ionically conducting soda-lime-silicate glasses. J Non-Crystalline Solids 354:3952–3957
Behera B, Nayak P, Choudhary RN (2007) Study of complex impedance spectroscopic properties of LiBa2Nb5O15 ceramics. Mater Chem Phys 106:193–197
Jamalpour S, Ghahramani M, Ghaffarian SR, Javanbakht M (2021) Improved performance of lithium ion battery by the incorporation of novel synthesized organic-inorganic hybrid nanoparticles SiO2-poly(methyl methacrylate-co-ureidopyrimidinone) in gel polymer electrolyte based on poly (vinylidene fluoride). Polym 228:123
Ramya CS, Selvasekarapandian S, Hirankumar G, Savitha T, Angelo PC (2008) Investigation on dielectric relaxations of PVP–NH4SCN polymer electrolyte. J Non-Cryst Solids 354:1494–1502
Khalil R (2017) Impedance and modulus spectroscopy of poly(vinyl alcohol)- Mg[ClO4]2 salt hybrid films. Appl Phys A 123:422
Acknowledgements
Authors gratefully thank the department of physics, B V Raju Institute of Technology, Narsapur and department of physics, Osmania University, Hyderabad for experimental facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeedi, V.R., Ganta, K.K., Mallaiah, Y. et al. Influence of succinonitrile plasticizer on ionic conductivity, structural and dielectric properties of potassium-based PEO/PVdF blend polymer electrolyte. J Polym Res 29, 64 (2022). https://doi.org/10.1007/s10965-022-02912-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-02912-y