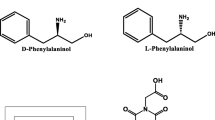
Abstract
Series of chiral oligomer ((S)-1-(S)-7)) based on the different proportions of achiral fluorophore unit (9, 9-dipropyl alkynyl fluorene (1a)) and chiral unit ((S)-2-(4-isopropyl-4, 5-dihydrooxazol-2-yl)-2-(prop-2-yn-1-yl) pent-4-ynenitrile (1b)) via Glaser coupling method were synthesized. The results showed that the fluorescent probe can be applied to the recognition of different enantiomers by changing the ratio of fluorophore to chiral group in the oligomer. The hydrogen bonding between chiral units and enantiomers in oligomers and large steric hindrance of rigid achiral units on conformation of the polymers may be the reasons for the different recognition abilities of oligomer with different ratios.






Similar content being viewed by others
References
Gal J (2019) Louis Pasteur, Chemical Linguist: Founding the Language of Stereochemistry. Helv Chim Acta 102:e1900098
Koichi M, Riku O, Tamihito N (2018) Chiral gas chromatography of 2,5-diketopiperazines following a ring-opening derivatization method for complete isomeric separation. J Chromatogr A 1566:118–123
Szakács Z, Sánta Z, Lomoschitz A et al (2018) Self-induced recognition of enantiomers (SIRE) and its application in chiral NMR analysis TrAC. Trends Anal Chem 109:180–197
Li Ping L, He Long P, Bao Hui Y (2009) Chiral sensor for enantiomeric purity of amines, amino alcohols and amino esters based on bis-cyclometalated Ir(III) complex using 1H NMR spectroscopy. Inorg Chim Acta 482:691–697
Guo HS, Kim JM, Chang SM et al (2009) Chiral recognition of mandelic acid by l-phenylalanine-modified sensor using quartz crystal microbalance. Biosens Bioelectron 24(9):2931–2934
Niu J, Rao L, Liu P et al (2018) Chiral recognition of dihydroxyphenylalanine enantiomers with (R)/(S)-2-Phenylpropionic acid grafted PProDOT electrochemical sensors. Synth Met 246:282–288
Li W, Liang J, Yang W et al (2014) Chiral Functionalization of Graphene Oxide by Optically Active Helical-Substituted Polyacetylene Chains and Its Application in Enantioselective Crystallization. ACS Appl Mater Interfaces 6(12):9790–9798
Raza S, Yong X, Deng J et al (2019) Optically Active Biobased Hollow Polymer Particles: Preparation, Chiralization, and Adsorption toward Chiral Amines. Ind Eng Chem Res 58(10):4090–4098
Zhang Y, Liu X, Qiu S et al (2019) A Flexible Acetylcholinesterase-Modified Graphene for Chiral Pesticide Sensor. J Am Chem Soc 141(37):14643–14649
Maeda K, Yashima E (2007) Dynamic Helical Structures: Detection and Amplification of Chirality. ChemInform 38(5):47–88
Gupta R, Gonnade RG, Bedekar AV (2016) Application of roof-shape amines as chiral solvating agents for discrimination of optically active acids by NMR spectroscopy: study of match–mismatch effect and crystal structure of the diastereomeric salts. J Org Chem 81:7384–7392
Huang HY, Bian GL, Zong H, Wang YB, Yang SW, Yue HF, Song L, Fan H (2016) Chiral sensor for enantiodiscrimination of varied acids. Org Lett 18:2524
Nieto S, Dragna JM, Anslyn EV (2010) A facile CD protocol for rapid determination of enantiomeric excess and concentration of chiral primary amines. Chemistry 16:227–232
Kumar A, Chae PS (2017) New 1, 8-naphthalimide-conjugated sulfonamide probes for TNP sensing in water. Sens Actuators B Chem 240:1–9
Wu YB, Guo HM, James TD, Zhao JZ (2011) Enantioselective recognition of mandelic acid by a 3,6-dithiophen-2-yl-9H-carbazole-based chiral fluorescent bisboronic acid sensor. J Org Chem 76:5685–5695
Banerjee S, Veale EB, Phelan CM et al (2013) Recent advances in the development of 1, 8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem Soc Rev 42:601–1618
D’Orazio G, Fanali C, Asensio-Ramos M, Fanail S (2017) Chiral separations in food analysis. TrAC, Trends Anal Chem 96:151–171
Dey G, Venkateswarulu M, Vivekananthan V, Pramanik A, Krishnan V, Koner RR (2016) Sub-picomolar recognition of Cr3+ through bioinspired organic–inorganic ensemble utilization. ACS Sens 1:663–669
Braña MF, Castellano JM, Morán M et al (1993) Bis-naphthalimides: a new class of antitumor agents. Anticancer Drug Des 8:257–268
Zhao F, Tian J, Wu X et al (2020) A near-IR Fluorescent Probe for Enantioselective Recognition of Amino Acids in Aqueous Solution. J Org Chem 85:7342–7348
Zhu YY, Wu XD, Gu SX, Pu L (2018) Free amino acid recognition: a bisbinaphthyl-based fluorescent probe with high enantioselectivity. J Am Chem Soc 141:175–181
Wang Q, Wu XD, Pu L (2019) Excitation of one fluorescent probe at two different wavelengths to determine the concentration and enantiomeric composition of amino acids. Org Lett 21:9036–9039
Wang CY, Zeng C, Zhang XL, Pu L (2017) Enantioselective Fluorescent Recognition of Amino Acids by Amide Formation: An Unusual Concentration Effect. J Org Chem 82:12669–12673
Zhang X, Chi L, Ji SM, Wu YB, Song P, Han KL, Guo HM, James TD, Zhao JZ (2009) Rational Design of d-PeT Phenylethynylated-Carbazole Monoboronic Acid Fluorescent Sensors for the Selective Detection of α-Hydroxyl Carboxylic Acids and Monosaccharides. J Am Chem Soc 131:17452–17463
Wu YB, Guo HM, James TD, Zhao JZ (2011) Enantioselective recognition of mandelic acid by a 3,6-Dithiophen-2-yl-9H-carbazole-based chiral fluorescent bisboronic acid sensor. J Org Chem 76:5685–5695
Wu YB, Guo HM, Zhang X, James TD, Zhao JZ (2011) Chiral Donor Photoinduced-Electron-Transfer (d-PET) Boronic Acid Chemosensors for the Selective Recognition of Tartaric Acids, Disaccharides, and Ginsenosides. Chem Eur J 17:7632–7644
Yashima E, Ousaka N, Taura D, Shimomura K, Ikai T, Maeda K (2016) Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem Rev 116:13752–13990
Wang SM, Yang Y, Shi XY, Liu LY, Chang WX, Li J (2020) Multiple Stimuli-Responsiveness Fluorescent Probe Derived from Cyclopolymers and Pyrene-Ended Ammonium Salts. ACS Applied Polymer Materials 2:2246–2251
Pu L (2017) Simultaneous determination of concentration and enantiomeric composition in fluorescent sensing. Acc Chem Res 50:1032–1040
Wu DT, Yu Y, Zhang J, Guo LL, Kong Y (2018) Chiral poly (ionic liquid) with nonconjugated backbone as a fluorescent enantioselective sensor for phenylalaninol and tryptophan. ACS Appl Mater Interfaces 10:23362–23368
Song FY, Wei G, Wang L, Jiao JM, Cheng YX, Zhu CJ (2012) Salen-based chiral fluorescence polymer sensor for enantioselective recognition of α-hydroxyl carboxylic acids. J Org Chem 77:4759–4764
Li Q, Peng YY, Han S, Lan TQ, Zhang J, Cao J (2020) Synthesis of Optically Active Graft Copolymers Carrying Polylactide Arms as Fluorescent Sensor for Recognition of Pyroglutamic Acid Enantiomer. ChemistrySelect 5:6549–6555
Miyabe T, Iida H, Banno M, Yamaguchi T, Yashima E (2011) Synthesis and Visualization of a Core Cross-Linked Star Polymer Carrying Optically Active Rigid-Rod Helical Polyisocyanide Arms and Its Chiral Recognition Ability. Macromolecules 44:8687–8692
Leophairatana P, Samanta S, De Silva CC, Koberstein JT (2017) Preventing alkyne–alkyne (ie, Glaser) coupling associated with the ATRP synthesis of alkyne-functional polymers/macromonomers and for alkynes under click (ie, CuAAC) reaction conditions. J Am Chem Soc 139:3756–3766
Bakhoda A, Okoromoba OE, Greene C, Boroujeni MR, Warren BJA, TH (2020) Three-Coordinate Copper (II) Alkynyl Complex in C-C Bond Formation: The Sesquicentennial of the Glaser Coupling. J Am Chem Soc 142:18483–18490
Acknowledgements
We thank Hunan Provincial Natural Science Foundation of China (No. 2016JJ6147) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Lan, T., Li, Q. et al. Chiral discrimination of enantiomers based on different interactions with alterable chiral oligomer. J Polym Res 28, 486 (2021). https://doi.org/10.1007/s10965-021-02832-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02832-3