Abstract
Derivatives of formyl pyrazole were synthesized by the reaction of acetophenone, 4-methyl acetophenone, 3-acetyl furan, 3-acetyl thiophen and phenyl hydrazine derivatives. The product was treated with Vilsmeier reagent producing different formyl pyrazole derivatives which were characterized by FT-IR, 1HNMR, Elemental analysis and Mass spectroscopy. The formyl pyrazole derivatives were reacted with chitosan to produce chitosan/ pyrazole Schiff base. These Schiff bases were characterized by FT-IR and TGA.The antimicrobial activity of chitosan/ pyrazol Schiff base (CSB) was evaluated against Gram-positive bacteria (Staphylococcus aureus and Bacillus cereus), gram negative bacterium (Escherichia coli) and fungus (Aspergillus niger). Results indicated agood inhibitory activity for CSB-14 when tested against B.cereus that gave inhibition zone of 7.5 ± 0.6 (mm), however CSB-18 gave a pronounced inhibitory activity against S. aureus and recorded 25 ± 2.0 (mm). All synthesized derivatives have no inhibitory activity against Gram negative E. coli. CSB-14, and CSB-15 exhibited inhibitory activity against tested A. niger that was used as a fungal model which gave 19 ± 0.9 and 18 ± 1.0 inhibition zone (mm) respectively. Thus, these results showed that, functionalization of chitosan with the hetero-cyclic compounds created biological activities of the synthesized derivatives; hence the synthesized pyrazole derivatives have not recorded any inhibitory activity before its immobilization onto chitosan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrazole is one of heterocyclic compounds that has two nitrogen atoms in five member ring [1], the presence of two nitrogen atoms in pyrazole nucleus exhibit its activity, while the nitrogen atom at position 1 can lose its proton during reaction with a base and nitrogen atom at position 2 is reactive towards electrophiles due to presence of a lone pair of electrons [2]. Due to the presence of pyrazole nucleus in different structures which provide it to have many applications such as anticancer, antibacterial, antidepressant, anti-inflammatory, antiviral, antifungal, antituberc-ulosis, antiviral and antioxidant [3, 4]. Chitosan is a natural polymer which consist of β-(1–4)-linked (acetylated) N-acetyl-2-amino-2-deoxy -D-glucose and (deacetylated) 2-amino-2-deoxy -D-glucose subunits [5, 6]. The main source of chitosan is a chitin which is found in abundance in nature [7, 8]. Chitin is obtained from crab and shrimp shell in the form of (N-acetyl glucose amine) polymer which is deacetylated to glucose amine unit with 40–50% conc. of NaOH [9, 10], unless the majority of polysaccharides that are behave as neutral or negatively charged in acidic medium, chitosan polymer behaves cationic nature in the this acidic medium thus, it forms a multilayer and electrostatic structure with negatively charged polymer [11]. The commercial interest toward chitosan and chitin is increased due to their biodegradability, biocompatibility and chelation with ions. It can be used as biomaterial for drug delivery, clinical applications, bioactive dressing, scaffold for the engineering of tissues, and anti-microbial to accelerate wounds healing [12,13,14]. Schiff bases are the compounds produced by condensation of carbonyl compound with primary amine [15], Schiff bases one of the most important classes in organic compounds, it can be used for numerous applications in pharmaceutical and medicinal fields such as anti-inflammatory. Furthermore, Schiff base have an application in analytical and in organic chemistry [16, 17]. It is shown that Schiff bases exhibit antibacterial, anticancer, antidepressant, analgesic anti-mycobacterial properties [18]. The anti-tumor and antibacterial activities have been reported to halo and nitro derivatives of Schiff base, Beta-lactam which is considered a derivative of Schiff base also showed antimicrobial activity and antifungal activities [19].
As discussed before, because of the biological activity of pyrazole, chitosan and Schiff base, we aimed to prepare new Schiff bases based on chitosan/pyrazole compounds and investigate the biological activities of the prepared compounds.
Experimental
Materials and methods
Materials
Acetophenone freezes under cool conditions with boiling point 202 °C, 4-methyl acetophenone with boiling point 226 °C, belongs to the class of organic compounds known as alkyl-phenyl ketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group, 3-acetyl thiophen also known as 1-thien-3-ylethanone or 3-acetothienone with boiling point 210 °C and melting point 60 °C which belong to the class of organic compounds known as aryl alkyl ketones.These are ketones have the generic structure RC(= O)R', where R = aryl group and R' = alkyl group. 3-Acetylthiophene was an extremely weak basic (essentially neutral) compound (based on its pKa)., 3-acetyl furan, which was known as 1-furan-3-ylethanone or 3-furanone with boiling point 84 °C and melting point 45- 47 °C which belong to the class of organic compounds known as aryl alkyl ketones.These are ketones have the generic structure RC(= O)R', where R = aryl group and R' = alkyl group, phenyl hydrazine appeared as pale yellow crystals, melting point 66 °F, with flash point 192 °F, auto-ignition temperature 345 °F. Soluble in alcohol, 2,4-Dinitrophenylhydrazine (DNPH) is a red to orange solid with melting point 198–202 °C and boiling point 378.6 °C. High molecular weight chitosan (more than 100 kDa) is soluble only in dilute acid with 82% degree of deacetylation were purchased from Sigma Aldrich, USA, Vilsmeier reagent (POCL3, DMF) while POCL3 that was known as phosphoryl chloride (commonly called phosphorusoxychloride) with the formula POCl3 with boiling point 105.8 °C. DMF that was known as dimethylformamide was an organic compound with the formula HCON(CH3)2, with boiling point 153 °C were purchased from LOBA CHEMI (Mumbai, India).
Instruments
FT-IR spectra were recorded in range 400–4000 cm− using Thermo Nicolet AVATAR 330 spectrophotometer and in presence KBr pellet method.1H-NMR data were recorded on a Varian Mercury VX-300 (300 MHz) NMR spectrometer. Thermo-gravimetric analysis was carried out on TGA-50H thermo-gravimetric analyzer, Shimadzu, Japan. Samples were heated up to 800 ∘C in a platinum pan with a heating rate of 10∘C/min, in N2 atmosphere of flow rate 25 mL/min.
Methods
General procedures for the synthesis of formyl pyrazole derivatives
The general method was exactly described as the following:
(Preparation of phenyl hydrazone derivatives): Acetophenone, 4-methyl acetophenone, 3-acetyl thiophene and 3-acetyl furan (0.1 mol) and phenyl hydrazine derivatives (0.1 mol) in a mixture of ethanol (30 mL) and acetic acid (1 mL) were refluxed in water bath for one h. The reaction mixture was cooled and filtered off. The solid product was washed with cold ethanol to produce the products that were outlined in Scheme 1.
(Synthesis of formyl pyrazole derivatives): Derivatives of hydrazones (0.1 mol) were added to Vilsmeier reagent (DMF and POCl3, 0.2 mol), dropwise with continuous stirring for one h in an ice bath. The reaction mixture was refluxed for 6 h at 70 °C then was poured onto ice/water mixture and neutralized by adding sodium hydroxide solution 5%. The precipitate was filtered off, dried to give the products that were shown in Scheme 1.
(General procedure for the synthesis of Chitosan based Pyrazole Schiff base): Chitosan (0.1 mol) was dissolved in 4% glacial acetic acid (100 mL) with stirring for 2 h, equivalent moles of each prepared formyl pyrazole were dissolved in (30 mL) boiled ethanol then added to chitosan solution drop wise with stirring, the reaction mixtures were refluxed for 6–7 h at 70 °C, cooled, the solid formed filtered off, washed with ethanol to remove unreacted aldehyde and dried to give the corresponding polymers that were outlined in Scheme 1.
Study of the antimicrobial activities:
The antimicrobial activity of formyl pyrazole derivatives and the prepared chitosan conjugated heterocyclic samples is assayed against some types of Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus), or negative bacterium (Escherichia coli) and fungus (Aspergillus niger). The antimicrobial test was carried out by agar diffusion method, where wells with diameter 6 mm were made. The prepared chitosan conjugated heterocyclic samples were dissolved in dimethyl sulfoxide at concentration of (10%). 50 µl of dissolved CSB derivatives (10 mg/ml) was loaded in medium agar well. The antimicrobial activity of the prepared samples against tested microorganisms was evaluated after 24 h of incubation at 37 °C for 24 h in case of bacterial organisms, however it was recorded in case of A. niger after 72 h of incubation at 25 °C. The samples diffuse through the wells, after that the inhibition zones were measured [20]. Amikacin and Nystatin were used as standard antibacterial and antifungal to compare with the synthesized CSB derivative.
Results and discussion
Characterization of phenyl hydrazone derivatives
The hydrazone derivatives were synthesized through refluxing of acetophenone, 4-methyl acetophenone, 3-acetyl thiophene and/or 3-acetyl furan with phenyl hydrazine derivatives in ethanol solution and few drops of acetic acid for one h [21], six different hydrazone derivatives (1–6) were obtained that were outlined in Scheme 1. These compounds were unstable for long time in air, thus we could not operate any characterization as FT-IR, NMR and elemental analysis for them.
For compound (1), 1-phenyl-2-(1-(p-tolyl) ethylidene)hydrazine that was characterized by (m.p. 100–103 °C, yield 83%), compound (2), 1-(2,4-dinitrophenyl)-2-(1-phenylethylidene)hydrazine that was characterized by (m.p. 225–230 °C, yield 81.5%), compound (3), 1-(2,4-dinitrophenyl)-2-(1-(p-tolyl)ethylidene)hydrazine showed (m.p. 250–255 °C, yield 82%), compound (4), 1-phenyl-2-(1-phenylethylidene)hydrazine that was characterized by (m.p. 104–106 °C, yield = 91%), compound (5) 1-phenyl-2-(1-(thiophen-3-yl)-ethylidene)hydrazine characterized by (m.p. 145–155 °C, yield 89%) and compound (6) 1-(1-(furan-3-yl)-ethylidene)-2-phenylhydrazine was characterized by (m.p. 139–141 °C, yield 87%).
Characterization of formyl pyrazole derivatives
Formyl pyrazole derivatives were synthesized through adding Vilsmeier reagent (DMF and POCl3) to hydrazone derivatives by ratio (2:1) respectively dropwise with stirring in ice bath for one h then refluxing at 70 °C for 6–7 h, six different formyl pyrazole derivatives (7–12) were obtained as shown in Scheme 1. The Vilsmeier-Haack reaction mechanism showed how the pyrazole ring was formed as shown in Scheme 2.
Compound (7), 1-phenyl-3-(p-tolyl)-1H-pyrazole-4-carbaldehyde was characterized by (m.p.118–120 °C, yield 81%) a yellow powder. 1H NMR (400 MHz, CDCl3), (δ, ppm) = 2.46 (3H, s, CH3), 7,3–8 (9H, m, Ar–H of benzene ring), 8.55 (1H, s, CH of pyrazole ring) and 10.079 (1H, s, CHO). FT-IR (KBr) cm−1: (1680 st, C = O, 1605 st, C = N). MS m/z: M = 262; Analysis Calcd. For.C17H14N2O: C, 77.84; H, 5.38; N, 10.68%; Found: C, 77.86; H, 5.44; N, 10.78%.
For compound (8); 1-(2,4- dinitrophenyl)-3-phenyl-1H-pyrazole-4-carbaldehyde: (m.p.240–243 °C, yield 79%) an orange powder. 1H NMR (400 MHz, CDCl3), (δ, ppm) = 7–8 (8H, m, Ar–H of benzene ring), 9.2 (1H, s, CH of pyrazole ring), 11.4 (1H, s, CHO). FT-IR (KBr) cm−1: (1614 st, C = O, 1507 st, C = N). MS m/z: M = 338; Analysis Calcd. For C16H10N4O5: C, 56.80; H, 2.97; N, 16.56%; Found: C, 56.86; H, 2.99; N, 16.59%.
While compound (9); 1-(2,4-dinitrophenyl)-3-(p-tolyl)-1H-pyrazole-4-carbaldehyde (m.p.230–240 °C, yield 75%), a red powder. 1H NMR (400 MHz, CDCl3), (δ, ppm) = 2.48 (3H, s, CH3),7–8 (7H, m, Ar–H of benzene ring), 9.2 (1H, s, CH of pyrazole ring), 11.4 (1H, s, CHO). FT-IR (KBr) cm−1: (1614 st, C = O, 1589 st, C = N). MS m/z: M = 352; Analysis Calcd. For C17H12N4O5: C, 57.96; H, 3.40; N, 15.90%; Found: C, 57.88; H, 3.49; N, 15.94%.
For compound (10); 1,3-diphenyl-1H-pyrazole-4-carbaldehyde: (m.p.142–143 °C, yield 91%), a white powder. 1H NMR (400 MHz, CDCl3), (δ, ppm) = 8.4 (1H, CH) of pyrazole, 7–8 (10H, m, Ar–H of benzene ring), 8.4 (1H, s, CH of pyrazole ring) and 9.96 (1H, s, CHO). FT-IR (KBr) cm−1: (1673 st, C = O, 1598, st, C = N). MS m/z: M = 248; Analysis Calcd. For C16H12N2O: C, 77.40; H, 4.87; N, 11.28%; Found: C, 77.45; H, 4.89; N, 11.32%.
For Compound (11); 1-phenyl-3-(thiophen-3-yl)-1H-pyrazole-4-carbaldehyde: (m.p.250–260 °C, yield 83%) a black powder. 1H NMR (400 MHz, CDCl3), (δ, ppm) = 8 (1H, s, CH) of pyrazole, 7–8 (8H, m, Ar–H of benzene ring) benzene ring and 9.3 (1H,s, CH),10 (1H, s, CHO)PPM of pyrazole nucleus. FT-IR (KBr) cm−1: (1675 st, C = O, 1597 st, C = N).Analysis Calcd. For C14H10N2OS: C, 70.56; H, 4.20; N, 11.75; S, 13.45%; Found: C, 70.62; H, 4.26; N, 11.78; S, 13.54%.
While compound (12); 3-(furan-3-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde: (m.p.250–255 °C, yield 87%), a green powder. 1H NMR (400 MHz, CDCL3), (δ, ppm) = 8 (1H,CH) of pyrazole, 7–8 (8H, m, Ar–H of benzene ring) and 9.3 (1H, s, CH), 10.133 (1H, s, CHO) ppm of pyrazole nucleus. FT-IR (KBr) cm−1: (1673 st, C = O, 1600 st, C = = N).
FT-IR spectral studies
From Table 1 and Fig. 1, the FT-IR spectral data were shown for 1-phenyl-3-(p-tolyl)-1H-pyrazole-4-carbaldhyde that were characterized by absorption bands due to aldehyde C-H, C = O and C = N in the regions 2850, 1680 and 1605 cm−1 respectively, the FT-IR spectra for 1-(2,4-dinitro-phenyl)-3-phenyl-1H-pyrazole-4-carbaldehyde were characterized by absorption bands due to aldehydic C-H, C = O and C = N in the regions 2926, 1614 and 1507 cm−1 respectively, another spectra of 1-(2,4-dinitrophenyl)-3-tolyl-1H-pyrazole-4-carbaldehyde are characterized by absorption bands due to aldehydic C-H, C = O and C = N in the regions 2918, 1614 and 1589 cm−1 respectively. In addition to, there are bands for (1,3) diphenyl-1H-pyrazole-4-carbaldehyde due to aldehydic C-H, C = O and C = N in the regions 2865, 1673 and 1598 cm−1 respectively.
The FT-IR spectra for 1-phenyl-3-(thiophen-3-yl)-1H-pyrazole-4-carbaldehyde are characterized by absorption bands due to aldhydic C-H, C = O and C = N in the regions 2364, 1675 and 1597 cm−1 respectively. The FT-IR spectra of for 1-phenyl-3-(furan-3-yl)-1H-pyrazole-4-carbaldehyde are characterized by absorption bands due to aldhydic C-H, C = O and C = N in the regions 2860, 1673 and 1600 cm−1 respectively.
1H-NMR spectral studies
The structures of formyl pyrazole derivatives were proved by 1H-NMR spectra which showed methyl proton at 2.46 ppm in compound (7), other peaks appear between 7–8 ppm corresponding (9H, m, Ar–H of benzene ring and 10H, m, Ar–H of benzene ring), in addition to it is shown proton single peak corresponding to CH group of pyrazole nucleus at 8.55 ppm for compound (7) and 8.4 ppm for compound (10) and another CHO proton appear as a single peak at 10 ppm for compound (7) and at 9 ppm for compound (10).
Also, by1H NMR spectra it was proved the structure of compounds (8 and 9). While, a single peak was shown at 2.46 ppm corresponding to methyl proton of compound (9), also, there are multiplet peaks between 7–8 ppm resulted from 8 and 7 H proton for aromatic rings respectively for each compound (8 and 9). In addition to there is a single peak at 9.2 ppm due to H proton of CH of pyrazole nucleus, also another peak appears as a single peak at 11.4 ppm resulted from 1H proton of CHO group, but increasing in de-shielding effect in CHO proton to 11 ppm due to effect of two nitro group in compounds (8 and 9).
For compounds (11, 12), It is reported that, presence of multiple peaks between 7–8 ppm due to 10 proton splitting in aromatic structure for each compound (11,12). Another single peak is shown at 9 ppm resulted from 1H proton of CH group in pyrazole nucleus, in addition to a single peak appear at 10 ppm corresponding to 1H proton of CHO group of compounds (11 and 12).
Characterization of chitosan based pyrazole Schiff base (CSB)
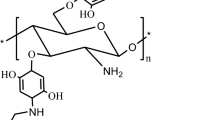
Formyl pyrazole derivatives were grafted to the amino group of chitosan forming different Schiff bases (13–18) that were represented in Scheme 1.
CSB-13 obtained from (conjugation of chitosan with 1-phenyl-3-(p-tolyl)-1H-pyrazole-4-carbaldhyde: 88% yield, faint yellow powder. FT-IR (KBr) cm−1: 3448 (st. OH), 1669 (st. CH = N). MS m/z: M = 421; Analysis Calcd. For C23H23N3O5: C, 72.49; H, 5.97; N, 11.82%; Found: C, 72.53; H, 5.99; N, 11.86%.
CSB-14 obtained from Conjugation of chitosan with 1-(2,4-dinitrophenyl)-3-phenyl-1H-pyrazole-4-carbaldhyde: 82% yield, orange powder. FT-IR (KBr) cm−1: 3488 (st. OH), 1608 (st. CH = N). MS m/z: M = 497; Analysis Calcd. For C22H19N5O9: C, 53.55; H, 4.18; N, 18.76%; Found: C, 53.58; H, 4.23; N, 18.80%.
CSB-15 obtained from (conjugation of chitosan with 1-(2,4-dinitrophenyl)-3-(p-tolyl)-1H-pyrazole-4-carbaldhyde: 86% yield, faint red powder. FT-IR (KBr) cm−1: 3450 (st. OH), 1605 (st. CH = N). MS m/z: M = 511; Analysis Calcd. For C23H21N5O9: C, 55.18; H, 4.82; N, 18.47%; Found: C, 55.24; H, 4.86; N, 18.53%.
CSB-16 obtained from (conjugation of chitosan with (1,3)-diphenyl-1H-pyrazole-4-carbaldhyde. 89% yield, faint yellow powder. FT-IR (KBr) cm−1: 3442 (st. OH), 1671 (st. CH = N). MS m/z: M = 407; Analysis Calcd. For C22H21N3O5: C, 71.68; H, 5.90; N, 12.13%; Found: C, 71.73; H, 5.15; N, 12.16%.
CSB-17 obtained from (conjugation of chitosan with (1-phenyl-3-thiophen-3-yl)-1H-pyrazole-4-carbaldhyde: 87% yield, brown powder. FT-IR (KBr) cm−1: 3451 (st. OH), 1647 (st. CH = N). MS m/z: M = 413; Analysis Calcd. For C20H19N3O5: C, 62.28; H, 4.29; N, 11. 34; S, 11.94%; Found: C, 62.31; H, 4.34; N, 11.38; S, 11.99%.
CSB-18 obtained from (conjugation of chitosan with (1-phenyl-3-(furan-3-yl)-1H-pyrazole-4-carbaldhyde: 85% yield, black powder. FT-IR (KBr) cm−1: 3441 (st. OH), 1665 (st. CH = N). MS m/z: M = 397; Analysis Calcd. For C20H19N3O6: C, 54.88; H, 5.29; N, 10. 44; %; Found: C, 54.91; H, 5.35; N, 10.40%.
From Fig. 1 and Table 2, the FT-IR spectra of chitosan (CS) showed an absorption band from 3431 cm−1, which corresponding to the stretching vibration of -OH and -NH groups, another absorption band appears at 2924 cm−1 corresponding to C-H stretching vibration, in addition to there was an absorption band at 1640 cm−1 due to acetylated amine or free amine on chitosan chain and another absorption band at 1071 cm−1 equal to the absorption frequency of C–O–C group of polysaccharide molecules [22].
The FT-IR spectra of different chitosan Schiff base derivatives (CSBs) showed an absorption band from 3441 to 3488 cm−1 corresponding to the stretching vibrations of –OH group for all Schiff base derivatives with increasing in frequency due to withdrawing effect of pyrazole derivatives on chitosan chain, another peaks characterized by absorption band in the regions of 1605 to 1669 cm−1 resulting from the stretching vibrations of (-CH = N-) imine group and this absorption band was absent for pure chitosan thus, the appearing of imine emphasizes the formation of Schiff base, in addition to, disappearance the absorption band around 1680 cm−1 corresponding to stretching vibration of aromatic aldehyde of formyl pyrazole which ensured the reaction of aldehyde group of formyl pyrazole with free amino group of chitosan. Another peak was observed in the regions ranges from 1405 to 1599 cm−1 due to the stretching vibrations of –C = C- of aromatic ring which is absent in pure chitosan. Thus, from the later spectral data the synthesis of Schiff base is emphasized.
TGA studies
Thermo-gravimetric analysis is employed to exhibit the thermal stability of the prepared polymer containing heterocyclic compound derivatives. From the later studies it was shown that, the TGA curve of pure chitosan shows two stages of weight loss is in the range from 47 to 450 °C, the first one in the range of 47–100 °C refers to loss of water molecules. The second degradation of pure chitosan started at 247 °C to completely degradation at about 450 °C [23,24,25]
From Fig. 2 it was shown that, chitosan/1-phenyl-3-(p-tolyl)-1H-pyrazole-4-carbaldhyde and chitosan/(1,3-diphenyl-1H-pyrazole-4-carbaldhyde respectively, showed a first degradation with total weight loss 90% between 159 till 290 °C and 129 till 325 °C attributed to the thermal decomposition of glucosidic linkage between glucose amine and N-acetyl glucose amine ring and pyranose ring of chitosan followed by a second degradation till 800 °C with weight loss 9.6% due to the degradation of pyrazole ring. while in case of chitosan bearing 1-(2,4-dinitrophenyl)-3-phenyl-1H-pyrazole-4-carbaldhyde, a thermal decomposition occur between 232–311 °C with weight loss 70% due to the thermal decomposition of glucosidic linkage between glucose amine and N-acetyl glucose amine ring and pyranose ring of chitosan followed by a second degradation till 800 °C with weight loss 10.4% due to the degradation of pyrazole ring. The thermal decomposition of chitosan / 1-(2,4-dinitrophenyl)-3-(p-tolyl)-1H-pyrazole-4-carbaldhyde was occurred between 255–315 °C with weight loss 74% due to the thermal decomposition of glucosidic linkage between glucose amine and N-acetyl glucose amine ring and pyranose ring of chitosan followed by a second degradation till 800 °C with weight loss 10% due to the degradation of pyrazole ring. The thermal degradation of chitosan/1-phenyl-3-(thiophen-3-yl)-1H-pyrazole-4-carbaldhyde occur at two steps, the first step from 27 to 570 °C respectively, with weight loss 46% resulted from adsorbed water loss, thermal decomposition of glucosidic linkage between glucose amine and N-acetyl glucose amine ring and pyranose ring of chitosan, and the second step between 570 to 800 °C with weight loss 12% due to the thermal degradation of pyrazole. While in case of chitosan/1-phenyl-3-(furan-3-yl)-1H-pyrazole-4-carbaldhyde the thermal studies showed.
decomposition occurred at four steps, the first step between 27 till 120 °C with weight loss 6% due evaporation of water molecules adsorbed or hydrogen bonded to chitosan and the second step from 120 till 240 °C with weight loss 18.692% attributed to degradation of glucosidic linkage between glucose amine and N-acetyl glucose amine, the third degradation from 240 to 330 °C with weight loss 17.2% due to degradation of pyranose ring and the last degradation occur from 330 till 800 °C with weight loss 31.881% corresponding to pyrazole moiety.
Antimicrobial activity
Heterocyclic pyrazole derivatives alone have not recorded any inhibitory activity before its immobilization onto chitosan. However, all synthesized Schiff base derivatives were tested in vitro against Gram-positive bacteria (staphylococcus aureus, Bacillus cereus) and Gram-negative bacteria (E.coli) and the fungus (Aspergillus niger).The results showed antibacterial and antifungal activity for all prepared Schiff base due to its inhibition for the two Gram-positive bacteria and poor activity against both E.coli bacteria and Aspergillus niger fungus. Table 3 data showed that, the higher antibacterial activity of CSB-18 against S. aureus other than the other tested compounds, and this might be due to the presence of furan ring. However, it is observed that the least anti-bacterial activity was recorded for CSB-13 and CSB-17 that have toluene and thiophene ring. Additionally, the data showed that the high antibacterial activity of compounds containing nitro groups.
Also, the compounds (CSB-13, CSB-14 and CSB-15) showed higher antibacterial activity that recorded inhibition zone 5 ± 0.4, 17.5 ± 1.2 and 20 ± 2.0 respectively, against Bacillus cereus (Gram-positive bacteria) than CSB-16, CSB-17 and CSB-18 that might be due to that CSB-13 and CSB-14 are containing tolyl and Nitro group, while CSB-16 and CSB-18 compounds exhibited medium effect that is lower than CSB-13 and CSB-14 and CSB-17 that containing thiophene group which performed the least effect. Concerning, Escherichia coli; all compounds do not give any effect against it that might be due to its high lipids contents and their cell wall chemical structure.
There was another proposal for the antimicrobial effects of the pyrazole-chitosan compounds are that chitosan can penetrate the nucleus of microbes and binds to DNA which inhibit the RNA binding site to DNA, thus the cell wall protein cannot be synthesized [26]. Results showed that these chitosan compounds have antifungal activities while, the studies ensure that, chitosan molecules can diffuse through fungal hyphae and inhibit enzyme that are responsible for fungal growth [27], in addition to the later has shown that chitosan molecules play an important role in inhibition of germ tube elongation, spore germination [28].
On the other hand, it is shown that the antifungal activity can be affected by chitosan Schiff base heterocyclic compounds derivative. While, it is observed that, both CSB-14 and CSB-15 showed the higher antifungal activity than the other compounds which possess nitro and phenyl group in CSB-14 and nitro and tolyl group in CSB-15, in addition to CSB-13 and CSB-16 compounds bearing phenyl and tolyl group that showed antifungal activity higher than CSB-17 and CSB-18 which bearing thiophenyl and furyl group respectively declaring the least activity as shon in Table 3. Although the synthetized heterocyclic compound derivatives were very reactive as antimicrobial agent but increasing of their hydrophobicity and decreasing their hydrophilic properties prevent them from penetrating the cell membrane of micro-organisms. Therefore, the immobilization of pyrazole derivatives with chitosan that have hydrophobic/ hydrophilic balance enhanced the pyrazole-chitosan derivatives to interact with the cell membrane. Moreover, the hydrophilic character of chitosan increased its selectivity to micro-organism.
Conclusion
Formyl pyrazole derivatives were synthesized through the reaction of acetophenone and phenyl hydrazine derivatives then the product is subjected with Vilsmeier reagent producing different formyl pyrazole derivatives which are characterized by FT-IR, 1H NMR, Elemental analysis and Mass spectroscopy, the later formyl pyrazole derivatives reacted by condensation with chitosan to produce chitosan/pyrazole Schiff base, These Schiff bases are characterized by FT-IR and the thermal properties such as DTA, DSC and TGA were studied, then the antimicrobial activity of chitosan/ pyrazole Schiff base was evaluated against some types of Gram-positive bacteria (staphylococcus aureus, Bacillus cereus), Gram-negative bacterium (Escherichia coli) and fungus (Aspergillus niger).
It is shown that, the biological activities of formyl pyrazole and chitosan against some types of Gram-positive bacteria (staphylococcus aureus, Bacillus cereus), Gram-negative bacterium (Escherichia coli) and fungus (Aspergillus niger) were increased by condensation reaction between formyl pyrazole and chitosan forming a Schiff base (-CH = N-) and the later containing a chitosan moiety having long carbon chain, the second moiety was formyl pyrazole carrying biological active derivatives, thus from the later it was shown that, these Schiff base have long carbon chain which led to increasing the hydrophobicity of molecules, thus, the chance of molecules passage through the cell membrane of microorganism was observed with high efficiency, in addition to decreasing their toxicity.
References
Kiyani H et al (2015) "Synthesis of new pyrazolyl-1, 3-diazabicyclo [3.1. 0] hexe-3-ne derivatives. J Mol Struct 1091:163–169
Bansal RK (2008) Heterocyclic chemistry, New Age International
Fustero S et al (2011) From 2000 to mid-2010: a fruitful decade for the synthesis of pyrazoles. Chem Rev 111(11):6984–7034
Ansari A et al (2017) Biologically active pyrazole derivatives. New J Chem 41(1):16–41
Kaur S, Dhillon GS (2014) The versatile biopolymer chitosan: potential sources, evaluation of extraction methods x 111(11): 6984–7034
Ifuku S (2014) Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 19(11):18367–18380
Rishabha M et al (2010) Preparation and evaluation of disintegrating properties of Cucurbita maxima pulp powder. Int J PhSci 2(1)
Chandur V et al (2011) Characterizing formulations containing derivatized chitosan with polymer blending. IJRPC 1(4):950–967
Morin-Crini N et al (2019) Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ Chem Lett 17(4):1667–1692
Fei Liu Xet al (2001) Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym 950- 967
Venkatesan J, Kim SK (2010) Chitosan composites for bone tissue engineering-an overview. Mar Drugs 8(8):2252–2266
Dang Y, Guan J (2020) Nanoparticle-based drug delivery systems for cancer therapy. Smart Materials in Medicine
Fadlaoui S et al (2019) Isolation and characterization of chitin from shells of the freshwater crab Potamon algeriense. Progress on Chemistry and Application of Chitin and its Derivatives 24:23–35
Tharanathan RN, Kittur FS (2003).Chitin-the undisputed biomolecule of great potential
Ashraf MA et al (2011) Synthesis, characterization and biological activity of Schiff bases. IPCBEE 10: 1–7
Sathe BS et al (2011) Synthesis characterization and anti-inflammatory evaluation of new fluorobenzothiazole schiff’s bases. Int J Pharm Res Dev 3(3):164–169
Udupi R (2012) Synthesis and biological screening of certain new triazole Schiff bases and their derivatives bearing substituted benzothiazole moiety. J Chem Pharm Research 4:1151–1159
Kajal, A., et al. (2013).Schiff bases: a versatile pharmacophore. Journal of Catalysts 2013
Meenachi, S. and Chitra, S. (2015).A review of chemistry and biological importance of Schiff base. ChemInform 46(13): pp 08- 18.
O’Brien TF et al (2019) Why surveillance of antimicrobial resistance needs to be automated and comprehensive. Journal of global antimicrobial resistance 17:8–15
Okhifo A et al (2019) Synthesis and Antimicrobial Evaluation of Some Simple Phenylhydrazones. J Chem Soc Nigeria 44(2):332–340
Chethan P et al (2013) Preparation of substitutedquaternized arylfuran chitosan derivatives and their antimicrobial activity. Int J Biol Macromol 59:158–164
Zawadzki J, Kaczmarek H (2010) Thermal treatment of chitosan in various conditions. Carbohyd Polym 80(2):394–400
Pereira FS et al (2013) Thermal studies of chitin–chitosan derivatives. Journal of thermal analysis and calorimetry 114(1): 321–327
Wanjun T et al (2005) Kinetic studies on the pyrolysis of chitin and chitosan. Polymer Degradation and Stability 87(3): 389–394
Raafat D et al (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74(12):3764–3773
Eweis M et al (2006) Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. Int J Biol Macromol 38(1):1–8
Romanazzi G et al (2018) Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties." Front Microbiol 9:2745
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Gharably, A.A., Kenawy, ER.S., Safaan, A.A. et al. Synthesis, characterization and application of chitosan conjugated heterocyclic compounds. J Polym Res 29, 141 (2022). https://doi.org/10.1007/s10965-021-02672-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02672-1