Abstract
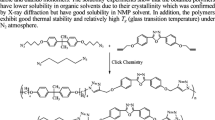
A novel class of clickable perfluorocyclobutyl (PFCB) aryl ether polymers containing azido groups (APFCB polymers) is reported. The thermal cyclopolymerization reaction of 2-methyl-1,4-bis(1,2,2-trifluorovinyloxy)benzene was first optimized by DSC, GPC and 19F NMR, and the matrix polymer (PFCB-Me) with high average molecular weights (Mw = 13.61 × 104 g/mol) and molecular weight distributions (Mw/Mn = 1.68) was obtained. Then, a series of APFCB polymers with tunable azido contents were successfully prepared from the PFCB-Me polymer by bromination and azidation. Post-functionalization of APFCB polymers with sulfonate functionality was accomplished by Cu(I)-catalyzed azide-alkyne cycloaddition click chemistry. The prepared polymers were characterized by FT-IR and NMR spectra, while the thermal properties and solubility were also investigated. The APFCB polymers exhibit glass transition temperatures in the range of 67 °C to 73 °C and good solubility in common organic solvents. This work suggests that the designed APFCB polymers may be a promising versatile platform for developing new functional PFCB aryl ether polymers.







Similar content being viewed by others
References
Narayanan G, Farajidizaji B, Smith DW et al (2020) Semi-fluorinated aromatic ether polymers via step-growth polymerization of fluoroalkenes. In: Ameduri B, Fomin S (eds) Opportunities for fluoropolymers: Synthesis, characterization, processing, simulation and recycling. Elsevier, Amsterdam, p 1–25
Zhou J, Tao Y, Chen X, Chen X, Fang L, Wang Y, Sun J, Fang Q et al (2019) Perfluorocyclobutyl-based polymers for functional materials. Mater Chem Front 3:1280–1301
Jin J, Topping CM, Chen S, Ballato J, Foulger SH, Smith DW et al (2004) Synthesis and comparison of CF3 versus CH3 substituted perfluorocyclobutyl (PFCB) networks for optical applications. J Polym Sci Part A: Polym Chem 42:5292–5300
Wang J, Zhou J, Jin K, Wang L, Sun J, Fang Q et al (2017) A new fluorinated polysiloxane with good optical properties and low dielectric constant at high frequency based on easily available tetraethoxysilane (TEOS). Macromolecules 50:9394–9402
Sun Y, Wang M, Gong X, Seo JH, Hsu BBY, Wudl F, Heeger AJ et al (2011) Polymer bulk heterojunction solar cells: function and utility of inserting a hole transport and electron blocking layer into the device structure. J Mater Chem 21:1365–1367
Kim DJ, Chang BJ, Kim JH, Lee SB, Joo HJ et al (2008) Sulfonated poly(fluorenyl ether) membranes containing perfluorocyclobutane groups for fuel cell applications. J Membrane Sci 325:217–222
Marestin C, Thiry X, Rojo S, Chauveau E, Mercier R et al (2017) Synthesis of sulfonate ester and sulfonic acid-containing poly(arylene perfluorocyclobutane)s (PFCB) by direct copolymerization of a sulfonate ester-containing precursor. Polymer 108:179–192
Souzy R, Ameduri B, Boutevin B et al (2004) Synthesis and (co)polymerization of monofluoro, difluoro, trifluorostyrene and ((trifluorovinyl)oxy)benzene. Prog Polym Sci 29:75–106
Kong L, Qi T, Ren Z, Jin Y, Li Y, Cheng Y, Xiao F et al (2016) High-performance intrinsic low-k polymer via the synergistic effect of its three units: adamantyl, perfluorocyclobutylidene and benzocyclobutene. RSC Adv 6:68560–68567
Zhou J, Fang L, Wang J, Sun J, Jin K, Fang Q et al (2016) Post-functionalization of novolac resins by introducing thermo-crosslinkable −OCF=CF2 groups as the side chains: a new strategy for production of thermosetting polymers without releasing volatiles. Polym Chem 7:4313–4316
Zhu Y, Chen H, He C et al (2011) Novel fluorinated polymers bearing phosphonated side chains: synthesis, characterization and properties. J Polym Res 18:1409–1416
Wang Y, Luo Y, Jin K, Sun J, Fang Q et al (2017) A spiro-centered thermopolymerizable fluorinated macromonomer: synthesis and conversion to the high performance polymer. RSC Adv 7:18861–18866
Zhou Y, Qing FL et al (2008) Novel fluorinated poly(aryl ether)s derived from 1,2-bis(4-(4-fluorobenzoyl)phenoxy)-hexafluorocyclobutane. J Fluorine Chem 129:498–502
Qian G, Smith DW, Benicewicz BC et al (2009) Synthesis and characterization of high molecular weight perfluorocyclobutyl-containing polybenzimidazoles (PFCB-PBI) for high temperature polymer electrolyte membrane fuel cells. Polymer 50:3911–3916
Jia M, Li Y, He C, Huang X et al (2016) Soluble perfluorocyclobutyl aryl ether-based polyimide for high-performance dielectric material. ACS Appl Mater Interfaces 8:26352–26358
Liu H, Zhang S, Li Y, Yang D, Hu J, Huang X et al (2010) A novel perfluorocyclobutyl aryl ether-based graft copolymer via 2-methyl-1,4-bistrifluorovinyloxybenzene and styrene. Polymer 51:5198–5206
Liu H, Zhang S, Li Y, Yang D, Hu J, Huang X et al (2011) A novel fluorine-containing graft copolymer bearing perfluorocyclobutyl aryl ether-based backbone and poly(methyl methacrylate) side chains. J Polym Sci Part A: Polym Chem 49:11–22
Hong J, Bi L, Li S, Jia G, Zhu Y, Li G, Huang Z et al (2016) Design and synthesis of reactive polymers containing perfluorocyclobutyl (PFCB) and hydroxyl moieties for post-functionalization of PFCB aryl ether polymers. Polymer 93:37–43
Bi L, Hong J, Li S, Zhu Z, Zhu Y et al (2019) Post-functionalization of perfluorocyclobutyl aryl ether polymers with a novel perfluorosulfonated side chain precursor. J Polym Res. https://doi.org/10.1007/s10965-019-1782-9
Lu G, Jiang X, Li Y, Lv X, Huang X et al (2015) Synthesis and self-assembly of PMBTFVB-g-PNIPAM fluorine-containing amphiphilic graft copolymer. RSC Adv 5:74947–74952
Lu G, Liu H, Gao H, Feng C, Li Y, Huang X et al (2015) Construction of semi-fluorinated amphiphilic graft copolymer bearing a poly(2-methyl-1,4-bistrifluorovinyloxybenzene) backbone and poly(ethylene glycol) side chains via the grafting-onto strategy. RSC Adv 5:39668–39676
Wu J, Lund BR, Batchelor B, Dei DK, Liff SM, Smith DW et al (2015) Suzuki polycondensation and post-polymerization modification toward electro-optic perfluorocyclobutyl (PFCB) aryl ether polymers: Synthesis and characterization. J Fluorine Chem 180:227–233
Gauthier MA, Gibson MI, Klok HA et al (2009) Synthesis of functional polymers by post-polymerization modification. Angew Chem Int Ed 48:48–58
Chang B, Kim D, Kim J, Lee S, Joo H et al (2008) Sulfonated poly(fluorene-co-sulfone)ether membranes containing perfluorocyclobutane groups for fuel cell applications. J Membrane Sci 325:989–996
Kalaw GJD, Wahome JAN, Zhu Y, Balkus KJ, Musselman IH, Yang DJ, Ferraris JP et al (2013) Perfluorocyclobutyl (PFCB)-based polymer blends for proton exchange membrane fuel cells (PEMFCs). J Membrane Sci 431:86–95
DesMarteau DD, Martin CW, Ford LA, Xie Y et al (2001) Sulfonated perfluorovinyl functional monomers US Pat 6:(268):532
Gooray NF, Takei F, Tomoi M et al (2016) Electrolyte composition, solid electrolyte membrane, solid polymer fuel cell and manufacturing method for solid electrolyte membrane. US Pat 7:(037):614
Moses JE, Moorhouse AD et al (2007) The growing applications of click chemistry. Chem Soc Rev 36:1249–1262
Günay KA, Theato P, Klok H et al (2013) Standing on the shoulders of Hermann Staudinger: Post-polymerization modification from past to present. J Polym Sci Part A: Poly Chem 51:1–28
Qi Z, Gong C, Liang Y, Li H, Wu Z, Feng W, Wang Y, Zhang S, Li Y et al (2015) Side-chain-type clustered sulfonated poly(arylene ether ketone)s prepared by click chemistry. Int J Hydrogen Energ 40:9267–9277
Cheatham CM, Lee S, Laane J, Babb DA, Smith DW et al (1998) Kinetics of trifluorovinyl ether cyclopolymerization via raman spectroscopy. Polym Int 46:320–324
Mifsud N, Mellon V, Jin J, Topping CM, Echegoyen L, Smith DW et al (2007) First identification of biradicals during thermal [2π + 2π] cyclopolymerization of trifluorovinyl aromatic ethers. Polym Int 56:1142–1146
Png R, Chia P, Tang J, Liu B, Sivaramakrishnan S, Zhou M, Khong HS, Chan SO, Burroughes JH, Chua L, Friend RH, Ho PKH et al (2010) High-performance polymer semiconducting hetero structure devices by nitrene-mediated photocrosslinking of alkyl side chains. Nat Mater 9:152–158
He S, Liu L, Wang X, Zhang S, Guiver MD, Li N et al (2016) Azide-assisted self-crosslinking of highly ion conductive anion exchange membranes. J Membrane Sci 509:48–56
Acknowledgements
We would like to thank the financial support from National Natural Science Foundation of China (21764002) and Guangxi Natural Science Foundation (2017GXNSFAA198273).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, S., Wu, J. & Zhu, Y. Clickable perfluorocyclobutyl aryl ether polymers bearing azido groups: synthesis and post-functionalization. J Polym Res 28, 27 (2021). https://doi.org/10.1007/s10965-020-02369-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02369-x