Abstract
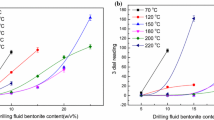
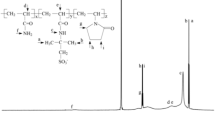
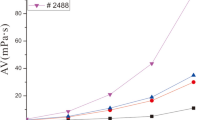
In this study, the inhibition property of an organosilicate polymer was investigated as an inhibitor for water-based drilling fluids. A novel polymer was prepared using 2-acrylamido-2-methylpropane sulphonic acid (AMPS), N, N-dimethyl acrylamide (DMAA), maleic anhydride (MA), and γ-methacryloxypropyl trimethoxy silane (MEMO) as the polymerisation monomers, named as ADMM. Also, the inhibition performance was evaluated by an immersion test, a linear swelling test, and a shale cuttings recovery test. Compared with that of the other inhibitors, the addition of ADMM can effectively inhibit the swelling of mud balls, resulting in the lowest swelling height of the drilling fluid. ADMM was characterised using a thermogravimetric analysis, a Fourier transform infrared spectrometer, a nuclear magnetic resonance instrument, a laser particle size distribution instrument, and a zeta potentiometric analyser. The results showed that the decomposition temperature of ADMM was 330 °C. The shale cuttings recovery of the 1.0% ADMM solution at 150 °C was 76.32%. The dynamic shear rise rate was as low as 160%. Furthermore, ADMM was adsorbed on the clay surface at many points to form a “protective film” of the polymer, thus inhibiting the hydration of the clay.










Similar content being viewed by others
Abbreviations
- ADMM :
-
Novel Organosilicate polymer prepared by AMPS, DMAA, MA, MEMO
- WBDF :
-
Water-based drilling fluid
- SEM :
-
Scanning electron microscopy
- mm :
-
Millimeter
- ppm :
-
Part per million
- XRD :
-
X-ray Diffraction
- AMPS :
-
2-acrylamido-2-methylpropane sulphonic acid
- DMAA :
-
N, N-dimethyl acrylamide
- MA :
-
Maleic anhydride
- MEMO :
-
γ-methacryloxypropyl trimethoxy silane
- AV :
-
Apparent viscosity, cp
- PV :
-
Plastic viscosity, cp
- YP :
-
Yield point, Pa
References
Pašić B, Gaurina Međimurec N, Matanović D (2007). Rudarsko-geološko-naftni zbornik 19:87
Liu X, Zeng W, Liang L, Lei M (2016). J Nat Gas Sci Eng 31:1
Striolo A, Cole DR (2017). Energ Fuel 31:10300
Ahmad, M.: 2014
Sondergeld, C. H.; Newsham, K. E.; Comisky, J. T.; Rice, M. C.; Rai, C. S. SPE Unconventional Gas Conference, 2010
Mao H, Guo Y, Wang G, Yang C (2010). Rock Soil Mech 31:2723
Wilson M, Wilson L (2014). Clay Miner 49:127
Liu X, Zeng W, Liang L, Xiong J (2016). Petroleum 2:54
Gholizadeh-Doonechaly, N.; Tahmasbi, K.; Davani, E. SPE international symposium on oilfield chemistry, 2009
Xiong, K.; Ma, P.; Yong, F.; Qian, F.; Yang, R.; Meng, Y. IADC/SPE Asia Pacific drilling technology conference and exhibition, 2012
Qu Y, Lai X, Zou L, Su YN (2009). Appl Clay Sci 3:265
Zhong H, Qiu Z, Huang W, Cao J (2012). Appl Clay Sci 67:36
Balaban RD, Vidal ELF, Borges MR (2015). Appl Clay Sci 105:124
Shadizadeh SR, Moslemizadeh A, Dezaki AS (2015). Appl Clay Sci 118:74
An Y, Jiang G, Ren Y, Zhang L, Qi Y, Ge Q (2015). J Pet Sci Eng 135:253
Chen Y, Zhao Y, Zhou S, Chu X, Yang L, Xing W (2009). Appl Clay Sci 46:148
Wang, Q.; Zhao, Y.; Zhou, S.; Li, M.; Xing, W. Computer Distributed Control and Intelligent Environmental Monitoring (CDCIEM), 2011 International Conference on, 2011, pp 1157
Cao J, Meng L, Yang Y, Zhu Y, Wang X, Yao C, Sun M, Zhong H (2017). Energ Fuel 31:11963
Thuc C-NH, Grillet A-C, Reinert L, Ohashi F, Thuc HH, Duclaux L (2010). Appl Clay Sci 49:229
Su J, Chu Q, Ren M (2014). J Polym Eng 34:153
Bai, X. D.; Yang, Y.; Xiao, D. Y.; Pu, X. L.; Wang, X., J Appl Polym Sci 1322015
Ma XP, Zhu ZX, Shi W, Hu YY (2017). Colloid Polym Sci 295:53
Yanovska E, Vretik L, Nikolaeva O, Polonska Y, Sternik D, Kichkiruk OY (2017). Nanoscale Res Lett 12:217
Huang X, Shen H, Sun J, Lv K, Liu J, Dong X, Luo S (2018). Acs Appl Mater Inter 10:33252
Jain R, Mahto V, Sharma V (2015). J Nat Gas Sci Eng 26:526
Yang L, Jiang G, Shi Y, Yang X (2017). Energ Fuel 31:4308
Xu J, Qiu Z, Huang W, Zhao X (2017). J Nat Gas Sci Eng 37:462
Xu JG, Qiu ZS, Zhao X, Zhong HY, Li GR, Huang WA (2018). J Pet Sci Eng 163:371
Xiong Z, Tao S, Li X, Fu F, Li Y (2016). Petroleum 2:361
Patel, A. D.; Stamatakis, E.; Davis, E.; Google Patents: 2001
Specifications, A., American Petroleum Institute
Zhong H, Shen G, Qiu Z, Lin Y, Fan L, Xing X, Li J (2019). J Pet Sci Eng 172:411
Dhiman, A. S., Halifax: Dalhousie University2012
Wan T, Li RX, Wu DQ, Hu ZW, Xu M, Cheng WZ, Zou CZ (2014). Polym Bull 71:2819
Wu YM, Sun DJ, Zhang BQ, Zhang CG (2002). J Appl Polym Sci 83:3068
Mao H, Qiu Z, Shen Z, Huang W (2015). J Pet Sci Eng 129:1
Herron MM (1986). Clay Clay Miner 34:204
Anderson RP, Tosca NJ, Gaines RR, Mongiardino Koch N, Briggs DE (2018). Geology 46:347
An Y, Yu P (2018). J Pet Sci Eng 161:1
Barast G, Razakamanantsoa A-R, Djeran-Maigre I, Nicholson T, Williams D (2017). Appl Clay Sci 142:60
Cao C, Pu X, Wang G, Huang T (2018). Chem Tech Fuels Oil+ 53:966
Chu Q, Luo P, Zhao Q, Feng J, Kuang X, Wang D (2013). J Appl Polym Sci 128:28
Zhong H, Qiu Z, Huang W, Cao J (2011). J Pet Sci Eng 78:510
Theng, B. K. G., Formation and properties of clay-polymer complexes; Elsevier, 2012
Liu, L.; Pu, X. L.; Rong, K. S.; Yang, Y. D., J Appl Polym Sci 1352018
Liu X, Zhao S (2008). J Appl Polym Sci 108:3038
Acknowledgments
This research was financially supported by Petro China Innovation Foundation (Grants 2018D-5007-0306), Joint Funds of the National Natural Science Foundation of China (No. U1762212), CNPC Science and Technology Project (No. 2018A-3907).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, F., Sun, J., Dai, Z. et al. Organosilicate polymer as high temperature Resistent inhibitor for water-based drilling fluids. J Polym Res 27, 107 (2020). https://doi.org/10.1007/s10965-019-1922-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1922-2