Abstract
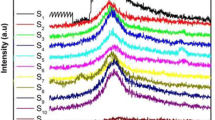
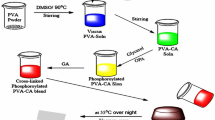
In this paper, 2-phosphonobutane-1,2,4-tricarboxylic acid (PBTCA), which is an organic phosphonic acids (OPA), is selected as the protic media to prepare phosphonated proton exchange membranes based on polyvinyl alcohol (PVA) by solution casting technique. The obtained PVA membranes were characterized using fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA), and wide angle X-ray diffraction (WAXD). The proton conductivities and the methanol permeabilities through the membranes were investigated in terms of various OPA content. The proton conductivity of the PVA membrane measured at room temperature was close to that of the nafion-117 membrane measured under the same test conditions (1.02 × 10−3 S·cm−1). But in the low relative humidity (50%), the proton conductivity of the phosphonic proton exchange membrane has a conductivity of up to 8.17 × 10−5 S·cm−1, which was significantly higher than that of the sulfonate proton exchange membrane. The methanol permeabilities of the PVA membranes were in the range of 10−7 to 10−6 cm2/s in the room temperature, depending on the OPD content. The thermal stability of the composite membrane was enhanced with incorporating of PBTCA by presenting high initial decomposition temperature.













Similar content being viewed by others
References
Jacobson MZ, Colella WG, Golden DM (2005) Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 308(5730):1901–1905
Steele BCH, Heinzel A (2001) Materials for fuel-cell technologies. Nature 414(6861):345–352
Jung WS (2018) Study on durability of Pt supported on graphitized carbon under simulated start-up/shut-down conditions for polymer electrolyte membrane fuel cells. J Energy Chem 27(1):326–334
Sun X, Xu H, Zhu Q, Lu L, Zhao H (2015) Synthesis of Nafion®-stabilized Pt nanoparticles to improve the durability of proton exchange membrane fuel cell. J Energy Chem 24(3):359–365
Zhang H, Shen PK (2012) Recent development of polymer electrolyte membranes for fuel cells. Chem Rev 112(5):2780–2832
Carretta N, Tricoli V, Picchioni F (2000) Ionomeric membranes based on partially sulfonated poly(styrene): synthesis, proton conduction and methanol permeation. J Membr Sci 166(2):189–197
Lufrano F, Baglio V, Staiti P, Antonucci V, Arico' AS (2013) Performance analysis of polymer electrolyte membranes for direct methanol fuel cells. J Power Sources 243:519–534
DELUCA N, ELABD Y (2006) Nafion®/poly(vinyl alcohol) blends: effect of composition and annealing temperature on transport properties. J Membr Sci 282(1–2):217–224
Chang Y, Wang E, Shin G, Han J, Mather PT (2007) Poly(vinyl alcohol) (PVA)/sulfonated polyhedral oligosilsesquioxane (sPOSS) hybrid membranes for direct methanol fuel cell applications. Polym Adv Technol 18(7):535–543
Paddison SJ (2003) Proton conduction mechanisms at low degrees of hydration in sulfonic acid–based polymer electrolyte membranes. Annu Rev Mater Res 33(1):289–319
Schuster M, Rager T, Noda A, Kreuer KD, Maier J (2005) About the choice of the Protogenic group in PEM separator materials for intermediate temperature, low humidity operation: a critical comparison of sulfonic acid, Phosphonic acid and imidazole functionalized model compounds. Fuel Cells 5(3):355–365
Mehta V, Cooper JS (2003) Review and analysis of PEM fuel cell design and manufacturing. J Power Sources 114(1):32–53
Li Z, He G, Zhang B, Cao Y, Wu H, Jiang Z, Tiantian Z (2014) Enhanced proton conductivity of Nafion hybrid membrane under different Humidities by incorporating metal–organic frameworks with high Phytic acid loading. ACS Appl Mater Interfaces 6(12):9799–9807
Allcock H (2002) Phenyl phosphonic acid functionalized poly[aryloxyphosphazenes] as proton-conducting membranes for direct methanol fuel cells. J Membr Sci 201(1–2):47–54
Kannan R, Islam MN, Rathod D, Vijay M, Kharul UK, Ghosh PC, Vijayamohanan K (2008) A 27-3 fractional factorial optimization of polybenzimidazole based membrane electrode assemblies for H2/O2 fuel cells. J Appl Electrochem 38(5):583–590
Jin Y, Diniz Da Costa JC, Lu GQ (2007) Proton conductive composite membrane of phosphosilicate and polyvinyl alcohol. Solid State Ionics 178(13–14):937–942
KIM D (2004) Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J Membr Sci 240(1–2):37–48
Gu S, He G, Wu X, Guo Y, Liu H, Peng L, Xiao G (2008) Preparation and characteristics of crosslinked sulfonated poly(phthalazinone ether sulfone ketone) with poly(vinyl alcohol) for proton exchange membrane. J Membr Sci 312(1–2):48–58
Cahan BD (1993) AC impedance investigations of proton conduction in Nafion™. J Electrochem Soc 140(12):L185
Pivovar BS, Wang Y, Cussler EL (1999) Pervaporation membranes in direct methanol fuel cells. J Membr Sci 154(2):155–162
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC 51503134, 51721091) and the State Key Laboratory of Polymer Materials Engineering (Grant 679 No. SKLPME 2017-3-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhiwei, W., Hao, Z., Qiang, C. et al. Preparation and characterization of PVA proton exchange membranes containing phosphonic acid groups for direct methanol fuel cell applications. J Polym Res 26, 200 (2019). https://doi.org/10.1007/s10965-019-1855-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1855-9