Abstract
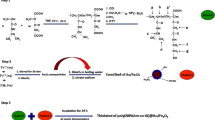
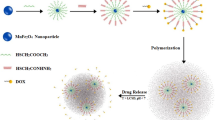
A magnetic polypeptide nanocomposite with pH and near-infrared (NIR) dual responsiveness was developed as a drug carrier for cancer therapy, which was prepared through the self-assembly of Fe3O4 superparamagnetic nanoparticles, poly(aspartic acid) derivative (mPEG-g-PDAEAIM) and doxorubicin (DOX) in water. Fe3O4 nanoparticles were prepared to provide the superparamagnetic core of nanocomposites for tumor targeting via chemical co-precipitation. The protonable imidazole groups of mPEG-g-PDAEAIM with a pKa of ~7 were accountable for the pH-responsiveness of nanocomposites. The photothermal effect of nanocomposites under the irradiation of NIR laser was induced via the interactions between dopamine groups of mPEG-g-PDAEAIM and Fe3O4 superparamagnetic nanoparticles to trigger the drug release. NMR, FT-IR, TEM, hysteresis loop analysis and MRI were utilized to characterize the materials. The DOX loaded nanocomposites exhibited pH-responsive and NIR dependent on/off switchable release profiles. The nanocomposites without drug loading (Fe3O4@mPEG-g-PDAEAIM) showed excellent biocompatibility while DOX loaded nanocomposites caused MCF-7 cells’ apoptosis due to the photothermal/chemotherapy combination effects. Overall, the pH and near-infrared dual responsive magnetic nanocomposite had a great potential for cancer therapy.








Similar content being viewed by others
References
Kievit FM, Zhang MQ (2011) Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res 44:853–862
Leslie-Pelecky DL (1996) Magnetic properties of nanostructured materials. Chem Mater 8:1770–1783
MacLachlan MJ, Ginzburg M, Coombs N, Coyle TW, Raju NP, Greedan JE, Ozin GA, Manners I (2000) Shaped ceramics with tunable magnetic properties from metal-containing polymers. Science 287:1460–1463
Reynolds CH, Annan N, Beshah K, Huber JH, Shaber SH, Lenkinsi RE, Wortmans JA (2000) Gadolinium-loaded nanoparticles: new contrast agents for magnetic resonance imaging. J Am Chem Soc 122:8940–8945
Shen LF, Stachowiak A, Hatton TA, Laibinis PE (2000) Polymerization of olefin-terminated surfactant bilayers on magnetic fluid nanoparticles. Langmuir 16:9907–9911
Shen LF, Laibinis PE, Hatton TA (1999) Bilayer surfactant stabilized magnetic fluids: synthesis and interactions at interfaces. Langmuir 15:447–453
Yu WW, Falkner JC, Yavuz CT, Colvin VL (2004) Synthesis of monodisperse iron oxide nanocrystals by thermal decomposition of iron carboxylate salts. Chem Commun 20:2306–2307
Sun SH, Zeng H (2002) Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc 124:8204–8205
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T (2011) Superparamagnetic iron oxide nanoparticles (spions): development, surface modification and applications in chemotherapy. Adv Drug Deliver Rev 63:24–46
Cheng FY, Su CH, Yang YS, Yeh CS, Tsai CY, Wu CL, Wu MT, Shieh DB (2005) Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 26:729–738
Fu L, Dravid VP, Johnson L (2001) Self-assembled (SA) bilayer molecular coating on magnetic nanoparticles. Appl Surf Sci 181:173–178
Wang YXJ, Hussain SM, Krestin GP (2001) Superparamagnetic iron oxide contrast agent: physicochemical characteristics and applications in MR imaging. Eur Radiol 11:2319–2331
Tian Y, Yu B, Li X, Li K (2011) Facile solvothermal synthesis of monodisperse Fe3O4 nanocrystals with precise size control of one nanometre as potential MRI contrast agents. J Mater Chem 21:2476–2481
Zhu XJ, Zhou J, Chen M, Shi M, Feng W, Li FY. Core-shell Fe3O4@NaLuF4:Yb,Er/Tm nanostructure for MRI, CT and upconversion luminescence tri-modality imaging. Biomaterials 2012;33:4618–4627
Hong RY, Feng B, Chen LL, Liu GH, Li HZ, Zheng Y, Wei DG (2008) Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem Eng J 42:290–300
Liu Y, Wang W, Yang J, Zhou C, Sun J (2013) pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J Pharm Sci 8:159–167
Chen F, Zhang J, Wang L, Wang Y, Chen M (2015) Tumor pHe-triggered charge-reversal and redox responsive nanoparticles for docetaxel delivery in hepatocellular carcinoma treatment. Nano 7:15763–15779
Rebek Jr J (1988) On the structure of histidine and its role in enzyme active sites. Struct Chem 1:129–131
Oh KT, Lee ES, Kim D, Bae YH (2008) L-histidine-based pH-sensitive anticancer drug carrier micelle: reconstitution and brief evaluation of its systemic toxicity. Int J Pharm 358:177–183
Liu Y, Ai K, Liu J, Deng M, He Y, Lu L (2013) Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater 25:1353–1359
Qiang W, Li W, Li X, Chen X, Xu D (2014) Bioinspired polydopamine nanospheres: a superquencher for fluorescence sensing of biomolecules. Chem Sci 5:3018–3024
Liu Y, Ai K, Lu L (2014) Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev 114:5057–5115
Black KCL, Yi J, Rivera JG, Zelasko-Leon DC, Messersmith PB. Polydopamine-enabled surface functionalization of gold nanorods for cancer cell-targeted imaging and photothermal therapy. Nanomedicine(Lond) 2013;8:17–28
Zheng R, Wang S, Tian Y, Jiang X, Fu D, Shen S, Yang W (2015) Polydopamine-coated magnetic composite particles with an enhanced photothermal effect. ACS Appl Mater Interfaces 7:15876–15884
Han J, Park W, Park S, Na K (2016) Photosensitizer-conjugated hyaluronic acid-shielded polydopamine nanoparticles for targeted photomediated tumor therapy. ACS Appl Mater Interfaces 8:7739–7747
Zhu CH, Lu Y, Chen JF, Yu SH (2014) Photothermal poly(N -isopropylacrylamide)/Fe3O4 nanocomposite hydrogel as a movable position heating source under remote control. Small 10:2796–2800
Ge R, Li X, Lin M, Wang D, Li S, Liu S, Tang Q, Liu Y, Jiang J, Liu L, Sun H, Zhang H, Yang B (2016) Fe3O4@polydopamine composite theranostic superparticles employing preassembled Fe3O4 nanoparticles as the core. ACS Appl Mater Interfaces 8:22942–22952
Lin LS, Cong ZX, Cao JB, Ke KM, Peng QL, Gao JH, Yang HH, Liu G, Chen XY (2014) Multifunctional Fe3O4@polydopamine core-shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano 8:3876–3883
Wang X, Zhang J, Wang Y, Wang C, Xiao J, Zhang Q, Cheng Y (2016) Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 81:114–124
Wang J, Gong C, Wang Y, Wu G (2014) Magnetic nanoparticles with a pH-sheddable layer for antitumor drug delivery. Colloid Surface B 118:218–225
Wu H, Tang L, An L, Wang X, Zhang H, Shi J, Yang S (2012) pH-responsive magnetic mesoporous silica nanospheres for magnetic resonance imaging and drug delivery. React Funct Polym 72:329–336
Guo M, Yan Y, Zhang H, Yan H, Cao Y, Liu K, Wan S, Huang J, Yue W (2008) Magnetic and pH-responsive nanocarriers with multilayer core–shell architecture for anticancer drug delivery. J Mater Chem 18:5104–5112
Chandra S, Noronha G, Dietrich S, Lang H, Bahadur D (2015) Dendrimer-magnetic nanoparticles as multiple stimuli responsive and enzymatic drug delivery vehicle. J Magn Magn Mater 380:7–12
Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloid Surface B 75:1–18
Lalatsa A, Schatzlein AG, Mazza M, Le TBH, Uchegbu IF (2012) Amphiphilic poly(l-amino acids)-new materials for drug delivery. J Control Release 161:523–536
Obst M, Steinbuchel A (2004) Microbial degradation of poly(amino acid)s. Biomacromolecules 5:1166–1176
Yoon SR, Yang HM, Park CW, Lim S, Chung BH, Kim JD (2012) Charge-conversional poly(amino acid)s derivatives as a drug delivery carrier in response to the tumor environment. J Biomed Mater Res Part A 100A:2027–2033
Takeuchi Y, Uyama H, Tomoshige N, Watanabe E, Tachibana Y, Kobayashi S (2006) Injectable thermoreversible hydrogels based on amphiphilic poly(amino acid)s. J Polym Sci Pol Chem 44:671–675
Pitarresi G, Saiano F, Cavallaro G, Mandracchia D, Palumbo FS (2007) A new biodegradable and biocompatible hydrogel with polyaminoacid structure. Int J Pharm 335:130–137
Liu DE, Han H, Lu H, Wu G, Wang Y, Ma J, Gao H (2014) Synthesis of amphiphilic polyaspartamide derivatives and construction of reverse micelles. RSC Adv 4:37130–37137
An JH, Huynh NT, Jeon YS, Kim JH (2011) Surfacemodification using bio-inspired adhesive polymers based on polyaspartamide derivatives. Polym Int 60:1581–1586
Jiang T, Wang Z, Chen C, Mo F, Xu Y, Tang L, Liang J (2006) Poly(aspartic-acid) derivatives as polymeric micelle drug delivery systems. J Appl Polym Sci 101:2871–2878
Zhao D, Li B, Han J, Yang Y, Zhang X, Wu G (2015) pH responsive polypeptide based polymeric micelles for anticancer drug delivery. J Biomed Mater Res Part A 103A:3045–3053
Gong C, Shan M, Li B, Wu G (2016) A pH and redox dual stimuli-responsive poly(amino acid) derivative for controlled drug release. Colloid Surface B 146:396–405
Gong C, Lu C, Li B, Shan M, Wu G (2017) Dopamine-modified poly(amino acid): an efficient near-infrared photothermal therapeutic agent for cancer therapy. J Mater Sci 52:955–967
Kang HS, Yang SR, Kim JD, Han SH, Chang IS (2001) Effects of grafted alkyl groups on aggregation behavior of amphiphilic poly(aspartic acid). Langmuir 17:7501–7506
Wang J, Gong C, Wang Y, Wu G (2014) Magnetic and pH sensitive drug delivery system through NCA chemistry for tumor targeting. RSC Adv 4:15856–15862
Guo Z, Ni K, Wei D, Ren Y (2015) Fe3+-induced oxidation and coordination crosslinking in catechol-chitosan hydrogels under acidic pH conditions. RSC Adv 5:37377–37384
Habash RWY, Bansal R, Krewski D, Alhafid HT (2006) Thermal therapy, part 1: an introduction to thermal therapy. Crit Rev Biomed Eng 34:459–489
Acknowledgements
This work was funded by the Natural Science Foundation of Tianjin (14JCYBJC18100), NSFC (51203079) and PCSIRT (IRT1257).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Gong, C., Li, B. et al. A magnetic polypeptide nanocomposite with pH and near-infrared dual responsiveness for cancer therapy. J Polym Res 24, 122 (2017). https://doi.org/10.1007/s10965-017-1277-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1277-5